


A distinguished Canadian researcher, Dr. Marie-Eve Tremblay, joins the ME/CFS/long-COVID fray.
New data comes in – and new models of ME/CFS and long COVID pop up. That’s, of course, how it should work and that’s what happened in “Chronic inflammation, neuroglia dysfunction, and plasmalogen deficiency as a new pathobiological hypothesis addressing the overlap between post-COVID-19 symptoms and myalgic encephalomyelitis/chronic fatigue syndrome“.
The nice thing about this model is that it takes the same two, possibly key, factors – inflammation and microglial activation – that we’ve been talking about, it seems for ages, and adds a third, more recent one – plasmalogen deficiency – to it. In other words, the research seems to be building on itself, the pieces seem to be fitting together, and it’s producing more than the sum of its parts. We will see whether the model will stand the test of time, but it’s nice to see research findings cohere together in this way.
It’s also nice to see distinguished researchers get pulled into the ME/CFS/long-COVID orbit. If anyone knows the neuroglia or the microglia, it’s the senior author of this paper, Marie-Eve Tremblay. (Microglia are a form of neuroglia). Hailing from the University of Victoria in British Columbia (Canadians, note – this paper was entirely a BC effort), Tremblay has managed to publish over 100 papers on the microglia and the central nervous system over the past 13 years. This is actually her second shot at ME/CFS. Last year, she co-authored Herbert Renz-Polster’s neuroglial (microglial) hypothesis paper “The Pathobiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome:The Case for Neuroglial Failure“.
As with so many other hypotheses, this one starts off with inflammation; i.e. chronic immune activation that just won’t quit. The authors note that many things could be causing neuroinflammation, or an “inflamed brain” – viral invasion, inflammation in the body, an autoimmune reaction, vagus nerve, and autonomic nervous system problems, and interestingly, angiotensin II overload.
Plasmalogen deficiency has been found in several neurodegenerative diseases. Could it be a core player in ME/CFS and long COVID?
The great question that any legitimate hypothesis must be able to answer is how to account for the many different triggers that can spark the ME/CFS spectrum of diseases (which includes, of course, long COVID). That clearly means getting at some core, or fundamental, physiological factors that have the capability of undermining a person’s physiology at a deep level.
In that vein, the authors brought in Angus Mackay’s ME/CFS hypothesis that a key stress integrator in the brain called the hypothalamic paraventricular nucleus (PVN) has been damaged. Stressors of all kinds (infections, pain, emotional distress, exercise) all converge on the little PVN. (Note that two published hypotheses from two people with ME/CFS – Herbert Renz Polster and Angus Mackay – have already made it into this paper :)).
The authors posit that inflammation “primes” the immune cells of the brain – the microglia – putting them in a hyperexcitable (could we say catastrophizing-like) state where they see danger everywhere. The slightest hint that something’s wrong causes them to jump into action and start pounding the area with “bullets”; i.e. pro-inflammatory cytokines. Maybe think of them like jumpy soldiers who, hearing a branch crack, unload with their guns – destroying the forest around them – clearly an exhausting and destructive process.
Since the microglia or neuroglia in the brain could conceivably play a role in so many of the issues found in long COVID and ME/CFS (movement, autonomic nervous system, sleep, homeostasis, sensory gating, memory, mood, and cognition), these amped up, twitchy immune cells could explain much of what’s going on.
This is all pretty much old hat, but a couple of studies from NIH-funded ME/CFS research centers run by Ian Lipkin and Maureen Hanson have opened up a whole new slant on how this may all have started. Enter the lipids and the cellular membranes.
Could Damaged Lipids and Cellular Membranes Be a Core Feature of ME/CFS?
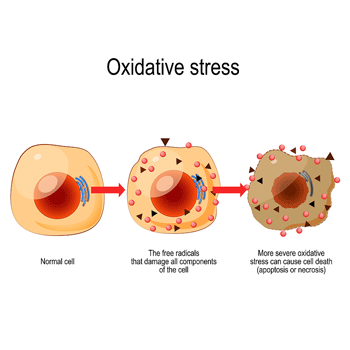
High levels of oxidative stress can damage cell membranes and even kill cells.
Talk about a core factor – the membranes, that cover our cells protect them from pathogens, toxins, and free radicals, and allow them to communicate with other cells, present the conduit through which all signals to the cells must pass. If the signal can’t get through to the cell, it can’t react to anything properly. It might as well be inert.
Plasmalogens are one the building blocks of the cell membranes that protect the cell. They contain polyunsaturated fatty acids (PUFAs), are powerful antioxidants, support cholesterol synthesis and transport, provide structural support for the cellular membranes, and play a key role in the “lipid rafts” that play a role in cellular signaling. They also appear to be low in ME/CFS.
The Peroxisomes
Since plasmalogens (PLs) are synthesized in little organelles in the cells called peroxisomes, the low plasmalogen levels found in ME/CFS suggest peroxisomal dysfunction is present. The authors believe the plasmalogens in ME/CFS are getting beaten up by high amounts of oxidative stress – an intriguing idea given that damaged mitochondria emit enormous numbers of free radicals.
Add in a recent finding that plasmalogens also play a role in mitochondrial health, and you potentially have quite a vicious circle forming – low plasmalogens impair mitochondrial health, leading to high levels of oxidative stress that further whack the plasmalogens, damage the cell membrane, and so on.
There’s also a peroxisome-mitochondria connection. The small, ubiquitous peroxisomes appear to punch far above their weight. Not only do they supply the building blocks for the cellular membranes, have anti-inflammatory properties, and reduce oxidative stress, they also break down crucial energy substrates for the mitochondria called long chain fatty acids. Recently peroxisomes have also been found to contain the “mitochondrial antiviral signaling protein (MAVS)” that plays a role in the innate or early immune response.
Peroxisomes are so effective at tamping down inflammation that dysfunctional peroxisomes in the brain have been associated with the accumulation of toxic byproducts and microglial activation.
Damaged peroxisomes in ME/CFS, then, could impair a host of core factors – the cell membranes, energy production, inflammation and protection from pathogens; i.e. it seems like just the kind of fundamental factor we’re looking for to explain complex, multisystemic diseases like ME/CFS, long COVID and similar diseases.
Breakthrough Paper
Several metabolomic studies have now found abnormalities across the range of lipids or fats found in cellular membranes. The authors noted that the big breakthrough for ME/CFS in this area may have come in a paper from Ian Lipkin’s group, which found disturbed peroxisomal lipid metabolism, reduced levels of the energy factor carnitine, and evidence of impaired mitochondrial functioning – and tied everything together in a nice bow.
The carnitine depletion does more than impair energy production. When peroxisomes are unable to process long-chain fatty acids, they leave behind long-chain triglycerides behind that are vulnerable to free radical attack – thus causing further peroxisome and mitochondrial damage; i.e. potentially producing a hot mess.
Given that, it was no surprise the Lipkin group study found that the peroxisome-related metabolites in ME/CFS were associated with increased fatigue scores.
Immune Exhaustion Too?
Health Rising recently published a blog suggesting that poor energy production in ME/CFS may be hobbling the innate immune system’s reaction to pathogens and toxins. That was intriguing given this interesting statement: “A disturbed mitochondria-peroxisome interaction could be the underlying factor for the suggested immune system exhaustion in ME/CFS patients.”
Evidence for that immune exhaustion in ME/CFS extends all the way from the T-cells of humoral or late immune response to the macrophages in the early or innate immune response. (Liisa Selin currently has an NIH grant to study T-cell exhaustion in ME/CFS.) Again, we see how a really fundamental factor – low plasmalogen levels and peroxisome dysfunction – could produce a wide array of effects.
Next, the authors go through the many ways that viruses interact with cell membranes, the possibility that the SARS-CoV-2 virus may be depleting plasmalogens, and the increasing evidence for fatty acid and plasmalogen depletion and mitochondrial issues in COVID-19 and long COVID.
The Gut
Then it was onto – what else? – a possible gut connection to all of this that involves a reduction in butyrate-producing gut bacteria in ME/CFS and long COVID that, in turn, impacts plasmalogen production.
The Brain
Next came the brain – Dr. Tremblay’s research focus – and a big hypothesis – that plasmalogen depletion plays a key role in many brain disorders including Alzheimer’s disease (AD), Parkinson’s disease (PD), Multiple Sclerosis (MS), depression, autism and Niemann-Pick type C disease, plus a new and intriguing possible connection – platelet-activating factor.
Dr. Tremblay recently wrote a review paper on the interactions between plasmalogens and platelet-activating factor (PAF) – an important player in the clotting process. That paper proposed that low plasmalogens trigger an increase in PAF and clotting and histamine release (!). The paper noted that increased PAF levels have been found in neuroinflammation, brain ischemia, and neurodegenerative diseases.
Next in this long, long paper came a detailed examination of the intersection between plasmalogens and estrogens, and the role low plasmalogens might play in the decidedly increased risk of ME/CFS in menopausal women. More sections covered the evidence that low plasmalogen levels play in neurodegenerative diseases.
THE GIST
- It’s always nice to see distinguished researchers get pulled into the ME/CFS/long-COVID orbit! In this case, it’s extra nice because in this case, it’s a microglial expert – Dr. Marie-Eve Tremblay.
- Her hypothesis paper takes the same two possibly key factors – inflammation and microglial activation – that we’ve been talking about, it seems for ages, and adds a third, more recent one – plasmalogen deficiency – to it, and comes up with a new hypothesis and a new (if expensive) treatment option.
- Tremblay and her British Columbia crew posit that inflammation “primes” the immune cells of the brain – the microglia – putting them in a hyperexcitable (could we say catastrophizing-like) state where they see danger everywhere. The slightest hint that something’s wrong causes them to jump into action and start pounding the area with “bullets”; i.e. pro-inflammatory cytokines.
- Plasmalogens are one of the building blocks of the cell membranes that protect the cell. They are also present in the organelles in our cells. Tremblay posits that low plasmalogen levels in the membranes of our cells are behind this mischief. Without healthy membranes to protect our cells from pathogens, toxins, and free radicals, and allow them to communicate with each other, our cells are pretty much toast.
- Plu,s the peroxisomes that produce the plasmalogens in the first place play a critical role in mitochondrial production. If both the peroxisomes and plasmalogens have gotten hit in ME/CFS and long COVID – and the latest research suggests they may have – that could explain much.
- The authors noted that the big breakthrough for ME/CFS in this area may have come in a paper from Ian Lipkin’s group, which found evidence of plasmalogen deficiency, balky peroxisome functioning, reduced levels of the energy factor carnitine, and impaired mitochondrial functioning – thus tying everything together in a nice bow.
- That plasmalogen deficiency could also explain the immune exhaustion found in ME/CFS. Plasmalogen deficiency has also been found in neurodegenerative diseases such as multiple sclerosis, Parkinson’s, and Alzheimer’s Disease.
- Plasma Replacement Therapy (PRT) has not yet been tested in ME/CFS or long COVID, but the authors believe it “could represent a valuable therapeutic tool against this emerging health condition (long COVID) affecting millions worldwide.” They suggest that “therapeutic plasmalogen” replacement may be key in treating these disorders.
- No PRT drugs, unfortunately, have been FDA-approved. Some plasmalogen-enhancing supplements are available. Dr. Goodenowe, a researcher/doctor who has been studying plasmalogens for over a decade, has created plasmalogen supplements that he says are 100-900 x’s more effective than others. At $199 and/or $99 per bottle, though, and with an early loading regimen required, the first month or couple of months would cost from $200-$400 a month.
- Whether plasmalogen supplements are the latest “wonder supplement”, or whether they could get at a core problem in ME/CFS and long COVID, is unclear.
- With academics like Dr. Tremblay proposing that plasmalogens play a key role in ME/CFS and long COVID, plasmalogen supplementation, and ultimately plasmalogen drugs, seems like something to keep an eye on.
Treatment
The treatment section noted the “compelling literature” suggested that low plasmalogen levels are common in metabolic and neurodegenerative disorders such as ME/CFS and long COVID as well as many others.
What to do? Boost plasmalogen levels with – what else – “plasmalogen replacement therapy” (PRT). The authors state that both in vitro (animal) and in vivo (lab) studies provide (again) “compelling” evidence that PRT that utilizes purified plasmalogens is able to dramatically increase plasmalogen levels in a variety of tissues and, most importantly, protect the neurons and glial cells against multiple kinds of injury – and therefore possibly reduce neuroinflammation. The authors also believe that PRT may help with aging, and chronic stress, and enhance responses to bacterial-produced toxins.
PRT has not yet been tested in ME/CFS or long COVID, but the authors believe it “could represent a valuable therapeutic tool against this emerging health condition (long COVID) affecting millions worldwide.” They suggest that “therapeutic plasmalogen” replacement may be key in treating these disorders.
They propose that the use of “naturally derived and synthetic analogs of PLs in combination with other nutraceuticals, such as dietary antioxidants and multimodal lifestyle interventions (e.g., acting on stress resilience, thermoregulation, metabolism) will be critical for the prevention, management, and treatment of multifactorial diseases including ME/CFS and post-COVID-19 syndromes.”
One review paper noted that three kinds of PRT exist. They include using metabolic precursors of plasmalogens, using plasmalogens derived from natural sources, and finally, and likely most effective, using synthetic analogs of plasmalogens. One synthetic plasmalogen-enhancing drug called PPI-1040 was given Orphan Drug Designation (giving it a leg up on the approval process) by the FDA for the treatment of Rhizomelic Chondrodysplasia Punctata (RCDP). I don’t know of any FDA approved plasmalogen drugs.
Neither of these papers mentioned plasmalogen supplements, but some are available.
Plasmalogen Supplements
According to the Top Supplements webpage, the ProdomeNeuro Plasmalogen supplement is designed to maximize absorption in the gut, thus producing plasmalogens that can cross the blood-brain barrier. Its producer, Dr. Dayan Goodenowe Ph.D., states it’s 100-900 times more potent than other supplements.
Goodenowe is a bit different from other functional medicine practitioners in that he actually does have a research background. He first proposed that plasmalogen deficiency played a role in Alzheimer’s Disease back in 2007, and over time, produced a dozen or so plasmalogen papers. A small 2010 study found that low plasmalogen levels were associated with reduced functioning in Alzheimer’s. His 2011 study found that an oil-derived plasmalogen precursor supplement was able to cross the blood-brain barrier and increase plasmalogen levels in rabbits.
His 2022 study of elderly people did not find that levels of amyloid plaques or neurofibrillary tangles were associated with reduced cognition, but reductions in some types of plasmalogens were. Only high brain levels of one type of plasmalogen were predictive of normal cognition in the elderly. A small Japanese study had a similar reduction, and plasmalogen levels are being actively studied in dementia.
In an interview, Goodenowe said he’d seen rapid results with plasmalogen therapy in dementia and even autism because, he thinks, plasmalogens are an incredibly basic molecule. Neuroinflammation, he believes, is having more fundamental effects than we think.
So far, so good. The supplement ProdomeNeuro his company Prodome Sciences produces has 900 mg of omega-3 plasmalogen oil per serving, but at $199 for sixty tablets with a recommended 1-3 month loading dose of 4-8 tablets a day, you’re looking at a minimum of $400 for the first month. The omega-3 plasmalogens are targeted at improving nerve functioning at the synapse and cognition.
At $99, another plasmogen supplement called ProdomeGlia Protector Plasmologen supplement with Omega-9 Plasmalogen Oil (ProdromeGlia™) and the same dosing regimen is less expensive ($200 for the 1st month or so) but still hardly cheap. Omega 9 plasmalogens are aimed at the white matter covering the nerves in the brain. They’re designed to tune your nervous system both inside the brain and in the body to ensure that your nerve conduction rates are fast and clear, and he referenced white matter degradation. This might be the one to try for ME/CFS and long COVID.
A less expensive supplement called LABO Nutrition NeuroRegain is derived from scallops. Another supplement called AKG, which contains a part of the amino acid glutamine (AKG), has been shown in animal studies to increase endogenous plasmalogen levels. It’s traditionally been used to increase muscle mass, and it may help with aging and cognition.
Conclusion
It was nice to see a distinguished researcher get excited enough at the latest ME/CFS and long-COVID findings to pen a paper proposing that researchers may be getting at the core aspects of the disease. Time, of course, will tell whether they’ve lit upon a really core part of these diseases.
Because plasmalogens play such a fundamental role in our cellular membranes, plasmalogen deficiency and problems with the peroxisomes which produce them have the potential to affect many systems and could cause many symptoms. While we don’t know the cause of the possible plasmalogen deficiency in ME/CF,S it may be possible to raise plasmalogen levels.
While plasmalogen drugs are not available, plasmalogen supplements – some of which are quite expensive – are. Are plasmalogen supplements simply the latest “wonder supplement” that’s being touted to be able to reverse all sorts of conditions, or could it really get at a core problem in ME/CFS and long COVID? For me, I would be shocked if any one supplement is enough, but with academics like Dr. Tremblay proposing that plasmalogens play a key role in ME/CFS and long COVID, plasmalogen supplementation seems like an intriguing, albeit expensive, idea.






Thank you Cort – another venue, we do not fully understand and needs to be explored!
Personally, I would assume that the dysregulated lipid remodeling is not specific to ME/CFS but rather a reflection of an impaired peroxisomal–mitochondrial crosstalk as postulated for viral reactivation and/or during significant oxidative stress (which both are part of the pathobiological matrix of ME/CFS but also of other diseases). This is why abnormal levels of membrane lipids are to be found in many neurodenerative disorders (as described in the paper).
Could the plasmalogen deficiency be a physiological bottleneck explaining ME/CFS symtoms? I doubt it. For this, the individual values in Che´s study show too much overlap with the healthy controls ((https://www.mdpi.com/1422-0067/23/14/7906 )). Also, the AUC values for distinguishing all ME/CFS patients from all controls ranged only between 0.514 and 0.738.
Because really, the most important question would be: is the plasmalogen deficit driving the clinical picture in ME/CFS or is it just another reflection of the many things that don´t work properly in ME/CFS? This is an important question because in the former case you´d expect possible improvement from supplementation, in the latter you wouldn´t. Here, it would be good to have a longitudinal picture of plasmalogen concentration during non-PEM and PEM phases, so far we do not have these values I think.
In the meantime I am sceptical of therapies that are advertised to work in Alzheimers, Parkinson´s, ME/CFS and others … ME/CFS is a specific disease outside the mold of neurodegenerative disorders . I´d just be surprised if the pathobiological bottleneck(s) were in the same place(s) in all these diseases.
I asked Avindra Nath whether ME/CFS had anything in common with the “big” neurodegenerative diseases like MS, ALS, etc. and he said no. They were very different yet here we have similar general findings such as low plasmalogen levels showing up and a researcher like Tremblay promoting plasmalogen replacement therapy. It’s hard to know what to make of it.
activation of the brain´s immune system (glia) could be the common denominator. Yet what “feeds” this activation could be way different
Microglia activate to all sorts of things though. Low oxygen can cause microglia activation
Please read below
Hello Dr. I read you believe cfs is outside neurodegenerative disorders. This new study with low plasmalogens you mentioned that there is too much overlapping with healthy and me/cfs patients. I am desperate at this point in my life. I am severe I am presently taking IViG with no change. I just started oxaloacetate do you have any other suggestions? Thank you
Cort, could you please add to the Gist an explanation of what plasmalogen is? I’ve not heard this word before. Too unwell right now to read the full article. Thanks as always for your work!
Thanks. This was a long one! I put this in the GIST: Plasmalogens are one of the building blocks of the cell membranes that protect our cells. They are also present in the organelles in our cells.
Thank you Cort!
Cort, are the plasmalogens generated naturally within the body?
Are they consumed in our food?
Why is there a shortage in the body – is that due to aging, illness, or all of the above?
Is there a way to encourage the body to naturally restore plasmalogens, or are there certain foods that provide plamalogens?
Thank you.
Hi Kate, From what I have read, plasmalogens are low at birth but increase dramatically over the first year of life. They are lost in old age; at the age of 70, levels are below 40% and falling fast. For people with neurodegenerative disease, this happens sooner.
We do ingest plasmalogens from meat and seafood and probably other sources. Beef and chicken are high in plasmalogens, but seafood offers plasmalogens with more omega 3 content. Raw milk from pastured cows may be another source and is a healing food.
Low levels of plasmalogens are correlated with neurodegenerative, psychiatric, and genetic disorders. I do not know if they have established a causal relationship yet.
There is one drug approved by FDA, and multiple non prescription replacement therapies. There are various sources – synthetic facsimiles of plasmalogens; natural sources of plasmalogens such as sea creatures; and precursors of plasmalogens which survive the stomach.
This last option may be the best way – Evidenced by fact that orally delivered Plasmalogens don’t cross the blood brain barrier!!!
Yet the therapy appears to be effective. Hypothesize that oral therapy is able to reach the brain somehow via the gut – which we know has a direct connection to the brain. It’s not getting there through the blood, but it is still restoring brain function somehow.
I think (and this is just my opinion) that the best things you can do from “within” the body is to eat really high quality, non processed, non toxic food; gentle movement without pushing yourself; and most important – manage and reduce stress! there is an inexpensive book on Amazon about the vagus nerve and how to train it towards parasympathetic (rest and digest) vs sympathetic (fight or flight) mode. Very simple little habits you can develop like how to breathe properly to maintain relaxation and calm. It is short and easy to read and can be purchased as book or audio.
https://www.amazon.com/Activate-Your-Vagus-Nerve-Sensitivities/dp/1612438741/ref=tmm_pap_swatch_0?_encoding=UTF8&qid=&sr=
We used to make Nexavir for Roger Sahni. Our relationship soured for a number of reasons so we bailed out. We currently make a plasmalogen that is rich in e-plas and DHA. Dr Goodenowe is happy to say our plasmalogens cannot work very well because of stomach issues and lack of the number of plasmalogens per dose. Logically this cannot stack up because his synthetic version, which he says is 100- 400 times more potent than our product, still requires a daily starting dose of 4 capsules. Yet the Japanese NeuroRegain still showed a measurably effect with just 1 mg daily. In his latest opinions he maintains that he invented Plasmalogens. Yet they were first discovered in 1924, thus he must be 150 years old by now. Enough said. I believe Plasmalogens will work on CFS and there are simple ways to dramatically increase any effect effect. We would prefer to introduce such plasmalogens enterically and we are in the process of building a capsule machine to capsule the very viscous plasmalogen. Stay tuned.
Regards
Joe Cave
Good luck Joe! Please tell us more when appropriate. 🙂
Great recognition of our Canadian researchers! Thanks Dr. Tremblay and team for helping inform the science. And thanks Cort for everything you do!
This is the obscure Biolkgy stuff I like to read Bout. Layman like me will never find on own
Yah for Canada! 🙂
Thanks once again for the “Gist”. Some days I’m just not up for understanding much.
It would be great if someone could get a crowdfunded supply for 6 months of the supplement and let us know if it helps.
Cheers
I hope Nath has found something that will lead to an objective starting point for more ME/CFS research and recognition. When will his study finally come out? If he too has not found anything concrete, we will continue to go in circles for decades.
Many lives have been destroyed by this nasty disease. The same goes for their loved ones. I know that many ME patients have taken their own lives. A sad observation. But don’t blame them. They are brave people.
Basically life is over if you have this disease. Especially if you have a more severe form. But also if you have a milder form. Why do we keep holding on to life? Fear of death? More attention should be paid to euthanasia.
We have to find ways to have as quality of lives as possible – not an easy thing at all. With regard to Nath – I think it will not be too long. My understanding is that the main paper is at the Journal and once it is published others are waiting in the wings.
My great question is what will the NIH do with it? Will they keep their promise and use it to funnel more funding into ME/CFS?
I am saddened by your post Gijs even though I understand and don’t disagree in some situations- I have certainly had the thought that I can’t go on
I don’t usually post but I can’t let this just go by- and I agree with Cort we have to find ways to have as good quality lives as possible
I am 70 years old, very sick, almost bedridden, and I have had CFS for 40 years so I have been very discouraged for sure
One thing I have tried very hard to manage is the despair- I am sharing my thoughts in the hope you will find it helpful and not give up
In my experience depression coexists for obvious reasons and can be treated- medication, a good therapist
Meditation and spiritual practice of some kind helps with the mental aspect also- hopelessness and despair are not impossible to overcome- I don’t mean to imply that our thoughts create our illness- I hate that baloney- but I do find that I can shift my mood consciously one day or one moment at a time
Relationships, family or wherever you can find some support and some love are important, asking for and receiving help is sometimes hard, people get isolated- but this happens to lots of people who don’t have a physical illness so it also can be looked at separately
I think separating out these threads helps because some things can be helped no matter how sick we are
I have felt ok in a weird way despite everything and that is my wish for you and all of us in this community
Thank you for posting and bless you for hanging in there and being of help to others!
Dear Gijs, I hope you are okay. Most of us hang in there on a wing and a prayer, to help us carry that desperate despair. We are stubborn enough to want to see the end of this disease. I know positivity from others can be annoying when we feel so ill and helpless; yet positive thoughts may, in fact, help us to heal. Why not take a dedicated approach and see what happens? Create some mantras on sticky notes, post them all over the place. Start with “TOMORROW is a great day to die.” Repeat…every day!
God bless and keep you, Gijs.
I heard Goodenowe on that podcast you posted a while back and got interested enough to buy a bottle of the Prodrome Glial. I didn’t notice any changes but I also didn’t take anywhere near the loading dose recommended now. So, I’m up for a second trial…going to do the Neuro form this time though because they state it is better for energy. If I notice anything, I’ll probably add the Glial back in too. I don’t see the point much of doing their testing as I’d rather apply that money to the products anyway considering there is little risk if I don’t actually need it.
One thing, I searched for a coupon code and NAT25 takes off 25%, at least for now. It would be so nice if they would give you a coupon code for the blog too!
Thank you for the fascinating article!
Maybe I should have asked! I didn’t think of that. Good luck and please let us know 🙂
Hi Remy,
Did you have any improvement after trying the supplements? How long did you take them at the loading dose, and are you still taking them? Thank you so much.
No, unfortunately I never did see anything from taking them. I honestly can’t remember how long I took the loading dose anymore, but I do feel confident I gave it a good trial the second time around. Now I just take a megadose of fish oil and astaxanthin. Good luck if you try!
Putting on the skeptic’s hat as usual here. The actual mechanism for low plasmalogen causing microglial reactivity is not explained in the paper as far as I can see. All I can gather is its association with chronic inflammation and neuro-degenerative diseases. So maybe Dr. Tremblay is assuming the chronic inflammation from the low plasmalogen level is causing the microglial reactivity, like in age-related degeneration?
There is no evidence of chronic inflammation in ME/CFS as far as I know. In fact, a paper from Lipkin’s group, I think, once said that the longitudinal inflammation level does not predict the symptom severity, noting that immune system is hyperactive for the first 3 years and then hypoactive for the rest. Maybe the low plasmalogen level that Dr. Lipkin found could explain the hyperactivity for the first 3 years but not ME/CFS itself.
If the low plasmalogen level only triggered the microglial hypersensitivity via chronic inflammation, replacing it won’t reverse the condition. It would be the same old “hit and run” case. (We already know chronic stress is one of the risk factors). If the low level in microglia itself is the cause, on the other hand, the therapy could potentially make a difference. I can’t tell which from the paper.
Our resident skeptic! 🙂
The actual mechanism for low plasmalogen causing microglial reactivity is not explained in the paper as far as I can see. – I agree. I think the potential connection comes because plasmalogens are produced by peroxisomes which also process fatty acids for the mitochondria – which appear to be low in ME/CFS.
Its also possible – altho I don’t know whether its mentioned or not – that weakened cellular membranes could be more susceptible to oxidative stress which could impact the mitochondria.
I looked up plasmalogens and mitochondria and there is potentially a direct link between plasmalogen deficiency and the mitochondria – https://www.life-science-alliance.org/content/2/4/e201900348
I don’t think anyone knows which is the chicken and which is the egg. While I can’t imagine that any supplement is enough to take on ME/CFS by itself I’m encouraged that a researcher of Tremblay’s stature thinks plasmalogens might help.
It may be a piece of the puzzle…Time will tell – as I think lipid work in ME/CFS is continuing.
Ha ha, I’ll accept the position.
Dr. Tremblay got my ears because I always described MECFS as an extreme premature aging — I’ve seen my mom suffering from PEM shortly before she passed away. Age-related microglial activation is directly caused by chronic inflammation. In MECFS, microglial cells are probably just hypersensitive without cause. I think Dr. Tremblay is seeing the connection between the two through the low plasmalogen. I think it’ll turn out to be more correlation than causation, but it would be great if her theory works out and patient finally get some therapy that works.
Unfortunately the plasmologen supplement comes to around $200 for one month..the glia version. Thats a chunk of change for a finding so new. Fascinating stuff though but waiting for more research. Would like to know more ways to boost it endogenous. Possibly via butyrate bacteria?
I wish we knew more about ways to calm the over excited microglial cells. None of my strategies seem to be working. Latest attempt was low dose doxycycline.
Have you tried Low Dose Naltrexone? LDN is an example of a relatively new class of therapeutic agents called glial cell modulators. Used a lot (and successfully) by people with Lyme disease and others for pain. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3962576/ I used it and it helped my headache but at a much lower level then suggested. Good article by Cort on it’s uses. https://www.healthrising.org/blog/2020/03/02/strangeness-low-dose-naltrexone-chronic-fatigue-fibromyalgia-dosing/
Yes but for some reason LDN didn’t do much for me.
Did you take it for a long time? It helped a little daily but it took about a 1.5 years for my headache to go away completely. Might be due to killing off/ repairing whatever is causing the inflammation in the first place? Right now it’s about the only thing to help the glial cells while we try and figure out the rest. I highly recommend doing genetic testing. My Dr recommended Genomic Insight. I found it very helpful and changed/ added supplements to help based on the info received. Blessings.
What LDN dosage did you use ? I titrated slowly over 1,5 years from 0.5 mg to 7.5 mg without so far much effect.
Tnx for sharing
I couldn’t tolerate the full dose recommended (4.5mg) Anything higher than 2.0 made me sick. Did you read the link in my post above? The woman that took 9mg before seeing results and has been on 6mg, twice a day for 8 years and is back to full health? It sounds like you can tolerate higher doses so it might be worth checking out. The post explains more about it. Hope it works for you!
T Allen, thank you for sharing the Genomic Insight source!
Have you tried Low Dose Naltrexone? It worked wonders for me. Calmed my neuro-inflammation down completely.
Have you tried low dose naltrexone?
An internet search found this
https://oilsfats.org.nz/wp-content/uploads/2016/02/Dawn-Scott-Nelson-talk-Nov-2016.pdf
That’s very informative. I wonder what that enzyme is that coukd aid absorption? I like mussels so will eat more. I’ve also read that bifidobacteria longum carry plasmologen. So boost that via pre and probiotics.
Cort, at the end of the article you refer to, “increased risk of ME/CFS in menopausal women”. Is this true?? Or did you mean to say “pre-menopausal”? Thanks for another great article
I am the co-corresponding author Yuru Deng, together with Dr. Tremblay of this review article (the final print just came out today), published in the Special issue: “Plasmalogens in neuroinflammation and neuroregeneration: from models to clinics” at Brain Research Bulletin:
http://www.sciencedirect.com/journal/brain-research-bulletin/special-issue/109K0Z66ZWX
For those who are interested in Plasmalgeons, there is another Special Issue “Solving the Plasmalogen Puzzle- from basic science to clinical application” at Frontiers:
http://www.frontiersin.org/research-topics/21748/solving-the-plasmalogen-puzzle—from-basic-science-to-clinical-application
I am happy to see the vivid discussion (no matter it is negative or positive, although I prefer the latter!) in your website which I have paid attention since last article of Ian Lipkin’s metabolomics study (2022), and their “One Sentence Summary: Plasma levels of plasmalogens are decreased in patients with myalgic encephalomyelitis/chronic fatigue syndrome suggesting peroxisome dysfunction.” has attracted my attention…
Plasmalogens, the lipids I have been after since 2000 and I call them cubic membrane (CM)-derived plasmalogens! I have never thought that plasmalogen deficiency may have a strong involvement in multiple inflammation-mediated diseases, including Alzheimer’s disease. I have been working on CM which often appear in cell organelles, especially mitochondria and ER in response to multiple stress and/or diseased conditions, including viral infection (doi: 10.3389/fcell.2021.630242)
Therefore I think I am qualified to say something about Plasmalogens, which are the amazing molecules indeed for me.
Yes. If I were those who have been suffering from ME/CFS and long-COVID, I will definitely give a try of using Prof. Fujino’s product (Plasmalogen BOOCS Special):
Company website: https://plasmalogenboocs.com
The reasons:
1. Prof. Fujino’s product is safe (my mother and I have been continuously taking for 4-5 years)
2. I have had no any COVID vaccination, and I just got my first COVID a month ago! My symptom is mild, and during this period I took double strength of Plasmalogen BOOCS Special and had fast recovery without any long-COVID development.
3. The price is not too expensive (even cheaper than a cup of coffee at Startbuks/per day), especially if one can get the product directly from Japan.
4. If one believes that ME/CFS has neuroinflammation behind playing the role. then he or she definitely may see the improvement in his or her ME/CFS illness.
5. my grandnephew is Autism boy since 3 years old, now he is 11 y/o. Early this year he has been taking Prof. Fujino’s product (I have consulted with Dr. Prof. Fujino) for more than 6 months, and has significant improvement according to his parents.
Since sPls is not the drug…I think many medical doctors will hesitate to recommend to their patients. For me, as long as it is safe and it works… it matters.
People tend to make thing too complicated!!!
I hope it helps.
Thanks so much Yuru for taking the time to comment – and thanks for the link to that product which I had not heard of. Continued good luck with your research 🙂
Thank you:-)
I am happy to be of help.
Yuru, Would low plasmalogens have an effect on norepinephrine storage, synthesis, or production? It sounds like low plasmalogens could cause increased permeability of membranes and thus “leakage” of norepinephrine from the norepinephrine storage granules in nerve terminals. Thoughts?
Thank you for the message. Be honest, I don’t know the answer.
But I will keep in mind and when I have some thoughts I will get back to you.
Looks like it opens a scam website? “Congratulations you won an iphone!” ?????
plasmalogenboocs.com
Cut and paste the “whole link”, and try it again?
It is ok from my side…
try this website:
https://plasmalogenboocs.jp/
I clicked the link of the product twice and I landed in a fake Amazon website.
Could you kindly check and advise ? I am actually interested in the product.
Thank you
https://plasmalogenboocs.com/
I click from my site, it appears ok?
You have to recognize this label when you do the on-line search:
Plasmalogen BOOCS Special
Brand: プラズマローゲンBOOCS
I also got the scam website repeatedly, including from the link on a Google search for the company. But here’s another site that sells the product: https://lifeirl.com/products/plasmalogen-boocs-special/ It’s out of stock, but you can see the description and packaging to compare with other options.
And here’s its entry on Amazon. It’s unavailable, but there are photos, a description, and a couple of videos.
Tee hee: https://www.amazon.com/PLASMALOGEN-BOOCS-Special-Plasmalogen-Boocs/dp/B097CX6QY8/ref=cm_cr_arp_d_product_top?ie=UTF8
Dear Tracy
I don’t know why the company link will lead to SCAM website. It is okay from my side.
You may try to this link:
https://www.amazon.com/NeuroREGAIN-Scallop-Derived-PLASMALOGEN-Alzheimers-Concentration/dp/B079GSQQJ8
NeuroREGAIN is the first generation of Prof. Fujino’s scallop-derived plasmalogen product.
I used to purchase this product for my mother before I could purchase directly from Japan through a friend who lives in Japan.
I found another link for this 2nd generation of product Plasmalogen BOOCS Special:
https://mirai-shop.ru/en/product/9343-1-3-1-40-1-3-1-1-2/
But I have not tried this website to purchase yet.
Hope it helps.
Try this website:
https://plasmalogenboocs.jp/
sophie rouse
sophie
Lipopolysaccharide causes systemic inflammation (body and brain) and brain microglial activation.
“Imaging robust microglial activation after lipopolysaccharide administration in humans with PET”
https://doi.org/10.1073/pnas.1511003112
Also, ‘humans are orders of magnitude more sensitive to endotoxin than other mammals, such as mice.’
Which is one reason why mice models of diseases don’t translate well to humans.
“Animal models of sepsis”
https://www.tandfonline.com/doi/full/10.4161/viru.26083
[There is post on ME/CFS and endotoxemia prior to this one]
Followup: Despite the cost, I tried ProdromeGlia for two months. I took one softgel upon waking and another at bedtime. Right away, my ‘restless legs’ at night (technically, ‘periodic limb movement disorder’) seemed to go away, my brain fog was significantly weaker, and there were signs of greater mental acuity beyond that. The effect didn’t change markedly over time, although on some days my restless legs returned, albeit more weakly.
Then I switched to NeuroREGAIN, in the hope that the effect would be stronger. Instead, from the first day, I went back to my previous condition: bad brain fog and restless legs at night. After six days without any improvement, I switched back to ProdromeGlia, which I continue to take.
This investigation could deliver substantial new insights into how to handle Long-term COVID and ME/CFS, thus paving up the possibility to successful medicines based on plasmalogen supplement.
I want to extend my heartfelt gratitude for this insightful and well-researched article on the potential of plasmalogens in treating ME/CFS and Long COVID. The depth of information provided is truly commendable, and it sheds light on an area that desperately needs more attention. As someone personally affected by these conditions, learning about emerging therapies and the science behind them brings a renewed sense of hope. Thank you for your dedication to educating and supporting the community with such valuable content. Your work is making a real difference!