

The “WASF3 disrupts mitochondrial respiration and may mediate exercise intolerance in myalgic encephalomyelitis/chronic fatigue syndrome” study is fraught with possibility. For one, it was published in The Proceedings of the National Academy of Sciences – one of the most prestigious science journals in the world.
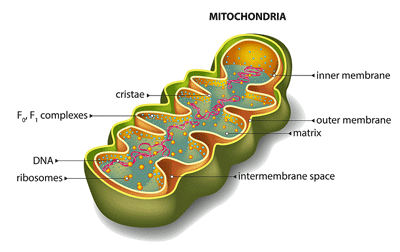
The mitochondria are back again in ME/CFS in…this time from NIH.
For another, it was produced by National Institutes of Health (NIH) researchers – a rarity. Usually, when we think of the NIH, we think of it as a funding source but the NIH also contains its own team of researchers. Avindra Nath’s Intramural ME/CFS study, for instance, is all being done “in-house” at the NIH. It’s the exception, though.
Look down the list of the authors of “WASF3 disrupts mitochondrial respiration and may mediate exercise intolerance in myalgic encephalomyelitis/chronic fatigue syndrome” and you’ll find they’re all from the NIH – and the vast majority are from an Institute – the National Heart, Lung and Blood Institute (NHLBI) – that hasn’t exactly been falling over itself to study or fund chronic fatigue syndrome (ME/CFS) research.
Lastly, the study boasts a nice Avindra Nath tie-in (Nath and Brian Walitt of the NINDS Institute are co-authors). The study used muscle samples gathered from Nath’s ME/CFS Intramural study and the authors thanked “the many individuals associated with the ME/CFS study … who made it possible for us to obtain de-identified samples of skeletal muscle”
The Chronic Fatigue Syndrome (ME/CFS) Connection
There’s a fascinating backstory. As reported in Science, “A protein that disrupts cells’ energy centers may be a culprit in chronic fatigue syndrome” (thanks to Brandon for the link). Paul Hwang, the senior researcher of the study, was interested in a family with a cancer-promoting mutation in a gene called TP53, one of which – a 38-year-old woman – had experienced increasing fatigue since coming down with infectious mononucleosis at age 16.
Extensive workups left her in a kind of gray area familiar to many. Suspected diagnoses included undifferentiated connective tissue disorder, systemic lupus erythematosus, and most recently, Sjogren’s syndrome (but apparently not chronic fatigue syndrome). Mitochondrial disease testing (mitochondrial genomic DNA sequencing and skeletal muscle biopsy studies) was unrevealing.
Looking for abnormalities in TP53-related pathways, they examined muscle tissue samples – and found very high levels of a protein called WASF3 in her samples but not in her siblings’.
Obscure ME/CFS Paper Provides Link

Suzanne Vernon’s pioneering effort on ME/CFS at the CDC in the mid-2000s ended up providing a key link.
What popped up in a literature search of WASF3 but a 2011 ME/CFS paper, “Meta-analysis of Chronic Fatigue Syndrome through integration of clinical, gene expression, SNP and proteomic data” with its own interesting backstory. The authors, who never published on ME/CFS again, were part of an extraordinary effort put together by Suzanne Vernon at the CDC.
While at the CDC, Vernon (who was later research director for Solve M.E. and is now research director for the Bateman Horne Center) produced one of the first large-scale efforts in any disease to integrate symptom, clinical, laboratory, gene expression, and proteomic data together.
The novel ME/CFS effort, called the C3 Computational Challenge, brought molecular biology, epidemiology, genomics, mathematics, engineering, and physics experts together to analyze the data gathered from 227 ME/CFS patients who’d undergone clinical evaluations, sleep studies, cognitive function, autonomic nervous system function, and extensive blood evaluations during a two-day hospital stay. Vernon said it was:
“a great opportunity to let loose the best and brightest minds in computational science on a really hard biological problem. The return was that we’d get a wealth of information from a first-of-its-kind study and dataset (the Wichita study) to advance our understanding of ME/CFS and maybe get people hooked on trying to solve ME/CFS.”
The results ended up filling an entire issue of the Pharmacogenomics journal in 2006 and prompted CDC leader Julie Gerberding to publicly declare that ME/CFS was a real and serious disorder that deserved more attention.
Next, Vernon enrolled CAMDA (Conference on Critical Assessment of Massive Data Analysis) in taking on the huge ME/CFS dataset. CAMDA, which has been in existence since 2000, invites researchers to probe “complex data sets, often featuring novel technological platforms, exceptionally large cohorts, and heterogeneous data sources and types.)
The short paper that provided the critical link to WASF3 and ME/CFS came out of the ME/CFS CAMDA competition. That paper – which highlighted 11 genes – stated “in particular, the gene WASF3 (aka WAVE3) possibly regulates brain cytokines involved in the mechanism of fatigue through the p38 MAPK regulatory pathway.”
That gene regulates the production of brain cytokines (think neuroinflammation) through the p38 MAPK pathway – which is involved in the central (i.e. brain-produced) mechanisms of fatigue. The paper, which never got much attention even in ME/CFS circles, has been virtually ignored – until now.
NIH Researchers Do Full Court Press on Mitochondrial Findings
It was remarkable watching these (apparently well-funded) researchers digging deeper and deeper into their findings.
Using a foot exercise, the NHLBI researchers twice assessed how well her phosphocreatine (PCr) levels rebounded after exercise and twice found that she had an unusually prolonged recovery period. The authors cited a 2004 ME/CFS study which apparently had similar findings.
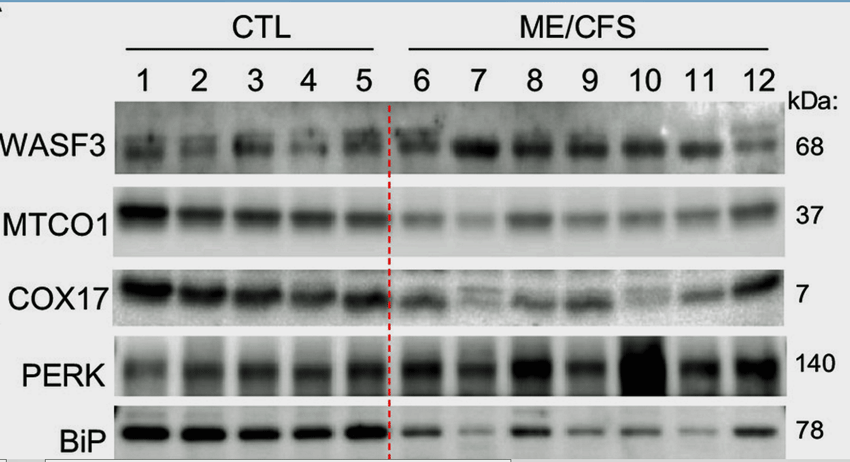
Note the high WASF3 and PERK levels and very low MTCO1 and BiP in the ME/CFS patients vs the controls on the left. The findings all fit together.
Next, they found that her muscle tissues demonstrated a lower oxygen consumption rate (read energy production) and a decreased ability to transfer electrons from complex III to complex IV in the electron transport chain of the mitochondria.
Then came a new finding – a 40% increase in WASF3 protein levels that was followed by a 34% decrease in the cytochrome oxidase enzyme that transfers electrons from complex 3 to 4 in the mitochondria.
THE GIST
- This study “WASF3 disrupts mitochondrial respiration and may mediate exercise intolerance in myalgic encephalomyelitis/chronic fatigue syndrome” stood out in a number of ways. It was published in the Proceedings of the National Academy of Sciences – one of the most prestigious science journals, and was produced by National Institutes of Health (NIH) researchers – a rarity.
- It began with a study into a cancer-promoting mutation that featured a 38-year-old woman who had experienced increasing fatigue since coming down with infectious mononucleosis at age 16.
- Her muscle tissues were found to have high levels of a protein called WASF3. When the researchers found a link to WASF3 in an obscure 2011 ME/CFS paper they were intrigued given her severe fatigue.
- That paper was one of many that resulted from a pioneering ME/CFS effort by Suzanne Vernon at the CDC which gave an enormous dataset derived from hundreds of ME/CFS to a group specializing in computational challenges. The small paper, however, had never attracted any attention until now.
- Various tests determined the woman’s cells were producing lower amounts of energy and, in particular, had a defect in their ability to transfer electrons from complex III to complex IV in the electron transport chain in the mitochondria.
- A 40% increase in her WASF3 protein levels associated with a 34% in the cytochrome oxidase enzyme sealed the deal: the transition from complex 3 to complex 4 in the mitochondria was toast.
- When they knocked down WASF3 levels in her cells her mitochondrial functioning improved. They reported “Collectively, these results showed that primary overexpression of WASF3 in cells leads to the disruption of respiration (energy production)”.
- Next, they created high WASF3 mice which produced high blood lactate levels during exercise – which has also been found in ME/CFS. The mice appeared normal but had a “remarkable 50% reduction in maximal running capacity”.
- ME/CFS patients from Avindra Nath’s big ME/CFS study were brought in and also found to have significantly increased WASF3 levels as well as evidence of problems in the transition from complex 3 to 4 in the mitochondria.
- Next, they went “upstream”: to see if the endoplasmic reticulum which regulate WASF3 production were functioning properly. They found a mess of endoplasmic reticulum problems and concluded that high levels of endoplasmic reticulum stress are present in ME/CFS.
- The outcome of all this was that a new mitochondrial abnormality that reduces energy production was found in ME/CFS. The authors stated that their finding provided a molecular explanation for the energy deficiency symptoms of exercise intolerance and postexertional malaise in a patient with chronic fatigue.“
- The study was also notable in how deeply this team dug even going to far as to uncover novel ways WASF3 affected the mitochondria and creating genetically altered mice.
- The study also involved Avindra Nath’s team. Early on reports suggested that Nath had found mitochondrial abnormalities in ME/CFS. Since then Nath has reported that his 5-year intensive study of ME/CFS was successful and will move the field forward and toward clinical trials. Time will tell but one could envision a major NIH effort with regards to the mitochondria in ME/CFS
- ER stress is found in other disorders and supplements and drugs are being assessed. The NIH team is now looking at ER stress-reducing drugs with the hopes of producing a clinical trial in ME/CFS.
“Collectively, these results showed that primary overexpression of WASF3 in cells leads to the disruption of respiration (energy production)”.
They took advantage of the genetic similarity between WASF3 in humans and mice to create transgenic (high WASF3) mice which produced particularly high WASF3 levels in the skeletal muscles. An exercise test indicated the high WASF3 mice produced higher blood lactate levels – which have been found in ME/CFS – and lower glycogen levels.
It got more interesting. As in ME/CFS, the mice appeared normal but showed a “remarkable 50% reduction in maximal running capacity”. Their grip strength, however, was not reduced, nor were the muscles structurally affected. The authors stated that the decrease in endurance appeared to result entirely from the mitochondrial problems found.
Next, they dug deep into WASF3 – which apparently had not been particularly well-studied with regard to mitochondrial functioning – and uncovered a number of new ways it affects the mitochondria. They concluded:
“overexpressed WASF3 can interfere with mitochondrial supercomplex formation…and (decrease) mitochondrial respiration (energy production)”.
Casting a Wider Net
With all that in hand, it was time to cast a wider net and bring in ME/CFS patients from Nath’s study. Not only did they find significantly increased WASF3 levels in the ME/CFS patients vs the healthy controls but also dramatically reduced cytochrome oxidase and MTO1 levels. (MTO1 is a protein expressed in high-energy-demand tissues such as the muscles.)
Because WASF3 is regulated by BiP (GRP78), an endoplasmic reticulum (ER) protein that checks proteins for quality control, the researchers went upstream to see if BiP levels were reduced – and they were even more significantly altered than WASF3. Plus, a marker of the endoplasmic reticulum stress called PERK was high.
With cytochrome oxidase levels negatively correlated with WASF3 and PERK levels, it looked like the researchers had uncovered a nice tight package of dysregulation – and now it was onto stressed out endoplasmic reticulum.
High Endoplasmic Reticulum Stress Response
The endoplasmic reticulum regulates protein folding, as well as protein transport and synthesis. Shape is everything in the body and if the proteins aren’t folded into their very intricate shapes correctly, they are unable to function. When levels of unfolded proteins build up, the endoplasmic reticulum stress response occurs. Viral infections and high levels of oxidative stress, among other things, can trigger an ER response.
ER stress has been found in neurodegenerative diseases, diabetes, metabolic syndromes, and cancer. Given the problem of misfolded proteins in Alzheimer’s Disease, it’s no surprise that ER stress and how to fix it is becoming a major emphasis in that disease. Several natural compounds and drugs (Berberine, Crocin, Bajijiasu, Echinacoside, Ginsenoside-Rg1, Salubrinal, Taurodeoxycholic acid) are being contemplated in AD. Metformin – which has been suggested for fibromyalgia, ME/CFS, and long COVID – is an ER stress response inhibitor.
The outcome of all of this was that the authors showed that “WASF3, induced by ER stress, disrupts the formation of respiratory supercomplexes and reduces mitochondrial oxygen consumption (energy production), providing a molecular explanation for the energy deficiency symptoms of exercise intolerance and postexertional malaise in a patient with chronic fatigue.”
They also showed – in the lab at least – that reducing the endoplasmic reticulum stress in the cells of an ME/CFS patient improved their mitochondrial functioning; i.e. they believe their finding could have “therapeutic implications for relieving fatigue symptoms in ME/CFS.”
They suggested that a similar scenario may be playing out in long COVID and other fatiguing rheumatic diseases such as fibromyalgia and rheumatoid arthritis.
- Check out the ME Association’s initial take on the study
Conclusion
There was no holding back on this study. It was a full-court press by some NHLBI researchers who have apparently become very interested in ME/CFS.
The authors were excited enough about the WASF3 finding to dig deeper and ultimately add substantial insights into the role WASF3 plays in the mitochondria. Its ability to disrupt the mitochondrial enzyme supercomplexes in ME/CFS was notable because genetic conditions that inhibit these supercomplexes are characterized by, you guessed it, exercise intolerance. The authors even proposed that high WASF3 levels in the brain may be contributing to mitochondrial problems and brain fog in ME/CFS.
This saga – which came together because of an (undiagnosed) ME/CFS patient in a cancer study and an obscure paper that came out of a novel and exploratory effort 15 years ago – demonstrates how serendipity can strike in the medical world.
Akiko Iwasaki, an immunobiologist at Yale School of Medicine told Science that the study was “very well done”. She cautioned that the suspect protein is likely “a piece of the puzzle, as opposed to explaining the whole disease” and could act as one of several “middlemen” between whatever sparks the illness and symptoms such as fatigue.
She’s probably right given the many other indications (disrupted autophagy, reduced fatty acid metabolism, high levels of intracellular calcium, complex V dysregulation, HHV-6 reactivation/mitochondrial fragmentation, citrate synthase deficiency, inborn errors of metabolism) that the mitochondria are involved in ME/CFS. What we really need is a full-court press on the mitochondria.
Pere Puigserver, a cell biologist at Harvard Medical School, told Science that it wasn’t clear whether ER stress was the problem or if the mitochondria were. Plus, he noted that WASF3 plays so many roles in the body that high levels could be causing other problems as well.
This small study will require further validation, but note that one critical part of the study gives us more hope than usual that these results may be on track. The study used muscle samples from ME/CFS patients in Avindra Nath’s Intramural study who went through an excruciatingly detailed process to ensure they only had ME/CFS. First checked over by a team of ME/CFS experts, they then underwent a week of intensive testing. These patients and the samples they left behind are like gold in a research world where ME/CFS is often considered a heterogenous, difficult-to-study disease.

Paul Hwang, the leader of the NIH effort, told Science he is looking at treatment options and hopes to do a clinical trial.
Avinda Nath and Brian Walitt from the ME/CFS Intramural study are clearly aware of the study. Early reports suggested that Nath might be finding mitochondrial abnormalities in ME/CFS. More recently, Nath reported that he’d found “consistent biological problems” and predicted the study was going to move the ME/CFS field forward “in a big way” and toward clinical drug trials. If both Nath and this team found mitochondrial abnormalities in ME/CFS, perhaps it’s time for the NIH to do the full-court press this issue needs and fund an RFA grant opportunity.
For his part, Paul Hwang, the leader of the effort, is looking at drugs to either reduce ER stress or reduce WASF3 levels to use in a clinical trial, and Director Walter Koroshetz of NINDS reported that Hwang and Nath are continuing to work together.






I wanted to point to the attention of readers that using artificial intelligence methods, ER Stress and its amelioration was identified in 2015 as a potential intervention using TUDCA which is discussed in the paper .More can be found in the following thread : https://twitter.com/lifeanalytics/status/1678408083834298368
Ha! “uncontrolled ER stress” – you were ahead of the times. :). And it was AI-generated back in 2015. I wonder what AI could do for us?
Efthymios by the ways is working on a data mining project with Solve ME. Thanks for chiming in on the blog.
Thankyou Cort for explaining this so well. I would like to see scientists investigate the correlation between WASF3 and Epstein Barr virus. I wonder if the OMF will use this research to improve theirs?
Sent you an email, Efthymios
What was the program/regiment you noted in your twitter link? Most was grayed out.
Are you still felling well?
Also, how long before you started feeling results?
Thanks.
@Bobby,
1) Some entries are grayed out because it is very likely that entries from this regimen will not work for other people. There were many patients who asked me to post the whole regimen and I will do so by also including necessary disclaimers and explanations.
2) Yes, I am able to live a normal life but I have to follow a specific regimen
3) It has been quite some time now, but if my memory serves me well I started feeling somewhat better in the first month. But I had to wait around 6-7 months to finally accept that I got out of the “vicious cycle” of ME/CFS
Hi Efthymios,
I would love also love to know the whole regimen please. I have been relentlessly researching for a way out for my daughter who was struck down with this brutal disease at 14! She was severely bed and housebound for over 4 years and has just started to come alive again after I put her on Dr Chia’s Equilibrant (4 tabs day) and has gone from bedbound operating at 10-15 percent to around 50-60 percent now! Can’t seem to get any further than this and although she is far better than she was, at nearly 19 and having missed out on her teenage years she would love to improve furthermore. I know there will be many pathways out of this mess and would like to look at your path a little more…many thanks…in gratitude …Kim
Dear Kim,
I have been a patient and I do understand that you would want to put an end to your daughter’s nightmare as soon as possible. The truth is that whatever worked for me most likely will not work for your daughter .
The best way forward is the collaboration of research teams backed with AI technologies. This will help us understand as soon as possible the complexities of ME/CFS and find much needed treatment.
Efthymios,
I have tried to take TUDCA but it gives me so much diarrhea I can’t leave the house. How do you take it?
What I did was that I started with 250 mg and reached 750 mg per day. This took place gradually, within 15 days. If you still have issues with the smaller dose , please talk to your doctor. TUDCA is another “version” of a medication called Ursodeoxycholic acid aka ursodiol : https://go.drugbank.com/drugs/DB01586
Thanks, I’ll try that.
Have you posted your regime? I am interested please.
Rather than just a virus in itself, do you think a too high dose of paracetamol/acetaminophen intravenously could also cause this liver damage? I got ill with ME/CFS after being hospitalised with acute rotavirus in 2015, and the hospital gave me Perfalgan intravenously, as far as I remember without taking my weight, and the per kg body weight dosing guidelines are different for adults under 50 kg… plus, I was already an adult with cerebral palsy/CNS impairment, and apparently acetaminophen can trigger hepatotoxity more easily in patients with existing CNS conditions? Any thoughts would be appreciated.
Thanks!
Inga
Hi Inga,
Yes, AI has identified a common trigger for a number of syndromes (including ME/CFS) which is liver injury. So, anything that can injure the liver (viruses, certain medications, toxic substances, during car accidents, etc) could be responsible in susceptible individuals. Interestingly , even traumatic brain injury (TBI) can injure the liver and TBI has many symptoms related to ME/CFS : https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4810977/
I mean, cerebral palsy is essentially traumatic brain injury at birth, and I have never really understood why cerebral palsy and other forms of TBI are treated as such distant and separate diagnostic groups, it makes no sense to me.
I am often a skeptic but this study is genuinely very promising.
By the way, Science deserves a lot of praise for its regular coverage of research on ME/CFS.
They were the only ones I’ve found so far to pick this up.
Science article is also linked in the Nature Daily Briefing.
Good to hear! Thanks.
Cort, do you know how many ME/CFS patients’ muscle tissues were eximined? How many of them were men? And what criteria were used?
It was 13 ME/CFS and I think 11 healthy controls. I’m not so worried about low number given that the samples came from Avindra Nath’s excruciatingly assessed ME/CFS patients in his intramural study which included a team of ME/CFS experts reviewing them.
Nath’s study included only ME/CFS patients with documented post-infectious triggers. It did include men but I don’t know which gender the samples came from.
Thx Cort, for this moment i am not impressed by their findings.
I like serendipity but in this case it was a cancer patiënt.
Me/CFS is not a mitochondria disease. A disfunction oke just like many disfunctions we have. What is starting this?
If ME/CFS is a mitochondrial disease I imagine it will add a new chapter on them.
Just wanted to point out the value this website has to p/w/ME/CFS.
Like many others, I saw this news start popping a few days ago, but what I could read was too brief or too complex.
Many thanks, Cort.
Thanks! Really a fascinating and hopeful finding in a quite a few ways. Who knows? Maybe Hwang and Nath are teaming up right now to take a deeper dive on the mitochondria in ME/CFS.
Excellent article. I could actually understand this complex topic. I’m relieved that the NIH is finally showing interest in ME/CFS. As an anecdote, I started taking Metformin 3 months ago for weight loss, and I’m showing a slight increase in energy.
TY for this important reporting, Cort.
Dr. Koroshetz from NINDS sent me this message yesterday:
“Yes, this is interesting. It actually is the result of a collaboration between Dr. Avi Nath the NINDS investigator on the NINDS ME/CFS protocol and Dr. Hwang in NHLBI who analyzed the muscle biopsies from the ME/CFS protocol patients. Dr. Nath emailed me today saying they are continuing to collaborate. There are two other papers that came out about findings studying Long Covid that might be relevant to ME/CFS more broadly. (attached) below”
https://www.science.org/doi/10.1126/scitranslmed.abq1533#:~:text=Overall, autopsy heart tissue from,and lymph node autopsy samples
https://www.biorxiv.org/content/10.1101/2023.02.09.527892v2
——-
So, it sounds like Nath & Hwang are indeed continuing to collaborate, FWIW.
“Dr. Nath emailed me today saying they are continuing to collaborate.” Music to my ears! Thank you.
(Maybe I should try emailing Koroshetz (lol). I always assumed he would be too busy. Pretty remarkable that the head of the huge NINDS Institute would reply…Good for him :))
FWIW, and I’m only n=1, but I’ve found that Whittemore and Koroshetz are generally very timely with communicating when I reach out. Pleasantly suprised, to be honest.
PS, this was Whittemore’s two cents when I reached out:
“The recent publication from Dr. Hwang’s group at NIH is a collaboration with NINDS and Dr. Nath as part of the NIH Intramural research on ME/CFS. I think the results are very interesting and potentially very important, but need to be replicated by another group and in a larger number of individuals with ME/CFS. I don’t have an update on the publication (ME/CFS Intramural Study). Last I heard it was still under review at the journal.”
[We’re at month 4 of review for whichever journal this went to for publication as it was submitted in late April). Hopefully it won’t be too much longer, and I know Brian V mentioned it could take 6-12 months]
Let’s hope that it’s 6 months and we’re on the cusp…Thanks!
I have been extremely unwell since I had Pfizer vaccination. I have fibromyalgia and CFS. I have enlarged lymph nodes from head to toe. My lymph system is blocked;or so it seems. I am looking for anyone who has had a similar problem or websites etc to explore this further. I live in Nova Scotia,Canada. It seems that there are so many places in the States to access experts in this field,clinics etc. I would travel anywhere,if I knew I could find someone to help me. Any reply would be appreciated !
The medical clinic RTHM, which specializes in treating patients with long-COVID and ME/CFS is also open to patients with “vaccine long haul” illness. This is from their website FAQ:
“Yes, we accept patients who developed Long COVID symptoms after vaccination as well as after infection! We are interested in the mechanisms behind ‘vaccine long haul’ and how they are similar and different from ‘infection-induced long haul.’ At RTHM, we feel it’s our duty to help the people who got the vaccine to help protect themselves and those around them and then suffered ill effects.”
I see a doctor at RTHM and I’ve been impressed with this knowledge and interest in the topic (I have had ME/CFS for 30 years). They solely do telemedicine. The downside is that they are quite expensive. If you have resources, I highly recommend them.
RTHM’s URL https://rthm.com/learn/
Good luck with finding what you need!
I am setting up a site for cdns including health clinics. One of comorbidities not being appropriately addressed is role CCI plays in the Symptomatology. See CCI novel theories section. Focusonhealing.ca
Look into the FLCC guidelines.
https://covid19criticalcare.com/protocol/i-recover-post-vaccine-treatment/
Covid vaccines have got to be the most evil thing perpetrated on the human race in decades. i warned everyone I knew NOT TO TAKE them. I just knew it was going to be horrible and dangerous. I know two healthy men who died from Myocarditis from getting the Jab. One was my next door neighbor and one was a brother of a girlfriend of mine. I also know a lady who developed multiple blood clots from the Jab and another lady who got shingles and a weird rash on her chin. They are gong to try an roll out another Covid booster soon and I hope and pray that no one falls for the propaganda again.
If you are having symptoms from the jab look into taking NAC and Nattokinase and doing a parasite cleanse using Ivermectin and fenbendazole and other parasite drugs. If you look into parasite drugs you will discover that there are many positive things they can do for you like killing cancer cells and treating MS. A Ketogenic diet, parasite drugs pulsed and adding in Nattokinase. Nattokinase will breakdown blood clots and fibrin and NAC will help your lungs and liver.
Be aware that they didn’t give everyone the actual Jab some people got a saline injection and some got a diluted injection and some got the real thing. They were experimenting on the whole world. I hope everyone wakes up to what is going on.
Hey I might have CFS but know a scam when I see and hear it;
Janice, hello…I too live in Canada.
I had 2 inch lumps all over my, mostly, upper body when I got sick decades ago.
I finally bought a copy of dr. Raymond perrins book.i started doing the lymph massage techniques…my lumps are not quit gone completely but close to gone some days. It’s about moving the toxins out of the body.
Jump on youtube..Dr Raymond Perrin has several videos on how to move lymph.you don’t really need the book.you may need to find a practitioner that he has trained. I tried to find one in my province of Sask. But there are none so I do my own lymph massages.its all about moving the lymph…but more importantly, in the correct direction
Janice, hello…I’ve replied to you but my reply ended up 3 or 4 replies down🤷♂️
Find it by my name
“Buckey”
Janice…I replied to you below
Again, these people are confused and calling exercise-inability exercise-intolerance. MECFSers who have been deconditioned for years can put out 100 Watt in 2-day CPET test. They don’t have exercise inability. They have exercise intolerance — they conk out the next day, for weeks, when similarly deconditioned subjects improve the performance.
It is exciting to me that you folks are getting to the bottom of what causes ME/ CFS. After having 10 bouts of CFS for the past 58 years, it thrills me to know we CFS suffers have hope of one day being free of this devastating fatigue experience. Keep up your good work!
“Therapeutic implications,” my two favorite words! Let’s hope this pans out into something useful, it seems very promising.
As always, Cort, you amaze me with how you can explain extremely complicated science in ways I can (mostly) understand. And I’m grateful for the hopeful news you share with us.
Thanks – For me, I wouldn’t nearly understand this stuff as well if I wasn’t writing out blogs on them. Thanks to everyone who supports Health Rising and allows me to do that. 🙂
This study certainly provides some hope. If only researchers would have followed up on the WAFS53 lead back in 2011.
Cort, any indication when Nath’s other studies may be released?
This is so interesting, thank you Cort for all you do!
I haven’t been diagnosed with ME/CFS but fibromyalgia.
I would love to be involved in their clinical trial, probably too late though.
I’m pretty sure I have mitochondrial disease because I lost two infants years ago from mitochondrial myopathy, cytochrome oxidase deficiency complex I and IV
It would be great to be involved in their clinical trial. Please let me know Cort if there’s a way to contact them
Thank you again for sharing this with us
Yes, that would have been nice but I would note that that paper really was obscure and took a quite experimental approach to the data. I think the techniques used may have been so new that the people who read it probably needed more convincing. I don’t even know if the mitochondrial link to WASF3 was known at the time. Plus since then we’ve had a good amount of work on the mitochondria and no one has ventured to look at this aspect. It truly came out of the blue.
For me, I didn’t cover it or remember that it had occurred.
This is so exciting. And.
At very least, we now have a catchy name for ME/CFS which will make people, researchers, journalists and governments sit up and take notice:
The ER Disease.
Ha! Brilliant!
Patient #3 here having taken part in Nath’s ME/CFS study (1st visit 2017, 2nd visit 2019). Pretty much ANYTHING is better than the insulting name “Chronic Fatigue Syndrome”. I’m 95% bed bound now and it’s not because I’m “fatigued”.
I am so deeply sorry to hear how much you are affected by ME/CFS. Thank you for taking part in the studies … in my eyes what you did is heroic.
I was not being entirely flippant with my suggestion. I think a name that has ER in it would make people think of Emergency Room … and make the connection that people with this disease are seriously ill and urgently need medical attention.
Bless you for taking part!
Thank you Cort. Finally something that induces real hope for realistic solutions. I hope I’ll see if in my lifetime. You even helped me get the gist of the science. I am almost 60, I live in the UK and I have had ME since I was 43. It has deteriorated from moderate to severe as I was a mother with 3 kids and no way of pacing.
Thank you Cort, this gives me hope.
I’ve had ME for 40 years at different levels, I’ve recently gone from very severe to severe thanks to LDN but it’s only the chance of a treatment or, better still, a cure that keeps me going.
Bless you. Keep on hoping. I think we are going to get somewhere soon xoxo
P38 MAPK has been identified to play a central role in some of the most advanced autism studies using SERT ala56 ASD mouse model. A drug MW150 has successfully completed safety trials in humans and probably will soon undergo human trials in ASD.
https://pubmed.ncbi.nlm.nih.gov/30297392/
https://pubs.acs.org/doi/10.1021/acs.jmedchem.9b00058
Wow, that’s also great to hear. I’m being evaluated for autism in October.
Cort, thank you!!! HOPE!
Dear Mr. Court Johnson, Let’s just take a moment to sincerely thank Mr. Johnson for giving us HOPE. You make a significant difference in our lives. You encourage us. Your dedication is palpable. Mahalo (thank you in the Hawai’ian language) for keeping my courage and fight going, Sir.
Katie Roberts-Williams ret. CCM, RN BSN MSN FNP
Thanks, Katie!
It was really encouraging to me that this study came from researchers from within the NHLBI which I believe may have been one of the Institutes which decided not to provide any funding for the ME/CFS research centers this time around.
The NIH has always said if it got good studies it would followup. Well, now it has a good study that its own researchers initiated. Time for some follow up so that we can really get a handle on what’s going on with the mitochondria.
This study comes at a good time as well as Trans NIH Working Group is going to come up with its strategic plan for ME/CFS by the end of the year.
This is some of the best news I’ve heard in awhile!
Thank you so much Cort! This is really exciting 🙂 It feels real.
Thank you Cort,
This is the most hopeful study I’ve seen yet.
My practical QUESTION, however, is this: Which drugs are researchers planning to use in upcoming clinical trials to reduce either WASF3 over-production or ER stress (or possibly both)?
Maybe I could induce my doctor to conduct a clinical trial on one (me).
I’m 80 and my PEM and muscle pain are getting rapidly worse.
I’m afraid I have no idea. Hopefully we will hear more at some point.
“The NIH team is now looking at ER stress-reducing drugs”
Rapamycin?
Very encouraging to again see researchers from other fields becoming aware of ME/CFS.
Yes – my thoughts as well. Very encouraging that these researchers – who had never studied ME/CFS before – really grabbed onto these findings!
So I asked ChatGPT “what supplements and drugs reduce endoplasmic reticulum stress?” It gave me this unranked list of “a few substances that have shown promise in reducing ER stress” (based on its dataset up to September 2021):
Tauroursodeoxycholic Acid (TUDCA): TUDCA is a bile acid derivative that has been studied for its potential to alleviate ER stress and protect cells from apoptosis (cell death) caused by ER stress. It has been investigated in various disease models, including neurodegenerative diseases.
[So taurine could be helpful, which I have been taking for years and seems to maybe help slightly.]
Resveratrol: Found in red wine, grapes, and some berries, resveratrol is known for its potential anti-inflammatory and antioxidant properties. Some studies have suggested that resveratrol might have a protective effect against ER stress.
Curcumin: Curcumin is a compound found in turmeric and is known for its anti-inflammatory and antioxidant properties. It has been studied for its potential to modulate ER stress and its associated cellular responses.
N-Acetylcysteine (NAC): NAC is a precursor to the antioxidant glutathione and has been investigated for its ability to reduce oxidative stress and potentially alleviate ER stress in various disease contexts.
Vitamin D: Emerging research has suggested a link between vitamin D deficiency and ER stress. Supplementing with vitamin D might have a positive impact on ER stress responses.
Omega-3 Fatty Acids: Omega-3 fatty acids, found in fatty fish and some plant sources, have been investigated for their potential to reduce ER stress and inflammation.
Quercetin: Quercetin is a flavonoid found in various fruits and vegetables. Some studies have indicated that quercetin might have a protective effect against ER stress-induced cell death.
Thank you for this very useful list! (And thanks to ChatGPT — plus your consulting it)
Thank you for running this question and getting this list.
I find this study very encouraging, it really feels like they are on to something here. I hope that this is going to be done at speed. If existing drugs can also be found to be helpful that would be a great bonus too. Thank you Cort,
Once again Cort, your ability to grasp and present this complex material to us in an understandable way continues to impress me. Researchers are making progress ! My take is that as these findings ( er dysfunction etc ) may relate to common diseases ie diabetes, Alzheimer’s….it will attract interest from researchers outside the ME/ CFS discipline……perhaps solving multiple problems as well !
That’s my take as well! ME/CFS seems to share more and more features with these other diseases. None of them is as debilitating as ME/CFS – so something unique is going on there – but the more disease features we share the better!
Excellent writing. The writer is an expert in ME/CFS ——not only explained the whole story in plain language, but also provided history of CAMDA that leads to the first clue of WASF3 in ME/CFS. Thanks!
So glad to get the CAMDA stuff in there. I still remember the C3 computational challenge and the Pharmacogenomics journal publications vividly. 🙂
Cort,
Do you know if elevated WASF3 was only found in subset of NIH intramural patients? Or was this finding for all study participants? I recall Brian Vastag and others were recalled for additional muscle studies after phase 2.
Thanks for your article.
I can’t tell from the stats that I see but I don’t believe WASF3 was elevated in all participants. Actually, the ER stress levels – which the researchers are focused on regarding treatment – were more altered than the WASF3 levels.
Interesting. What were the markers they looked at that indicated elevated ER stress levels? Also, any indication or hypothesis on what triggers ER stress and failure to return to pre infection state?
So mitochondrial disease characterused by PEM? Do pem is not unique to cfs?
Good question. There is certainly exercise intolerance in mitochondria disease. As to post-exertional malaise – the big payback for exerting oneself – I don’t know. The fact that it was the ME/CFS community that birthed the term PEM suggests to me that either it doesn’t exist or perhaps not in a big way.
I see thanks cort. Would you characterise the paper as a breakthrough? I wasn’t familiar with the mitochondria issues before but I had read that they weren’t the same type as actual mitochondrial disease
I think of it as a potential breakthrough – we need bigger studies to validate it. So many alterations in the mitochondria have been found in ME/CFS that maybe the bigger breakthrough might be a real effort by the NIH to figure out what’s going on in the mitochondria. Is there really something there or not – and if there is what is causing it and how important is it?
I see thanks again!
I have 2 types of mitochondria disease one is MTND4 other is MTND5. The first one is LHON Leber Hereditary Optic Neuropathy the other could be Leigh Syndrome both double copies.
I have the single mutations for acute myeloid leukemia maturation & I have the Li-Fraumeni gene.
I show the WASF3 in my raw data not sure where it is in the genetic genie results. I did the 23andme health ancestry DNA home saliva test.
I had a rare NET tumor removed in 2021stomach, it is in the literature tied to TP53 LI-Fraumeni but rare so is Hodgkins Lymphoma L & Non-Hodgkins.
My nephew had both when he was about 30 years old he is in remission.
This study began with the case of a 16 yr old who came down with mono which is EBV. EBV is the connection here that caused the mitochondrial problems. There’s other studies also that show a correlation between viruses and mito problems.
Mine started the same way. Mono at 16. I am now 62.
So how can I treat this now to see if anything works?
Hi Steve, I think we are a very mixed bunch and you would probably get a different response from everyone but I wanted to try and say what’s been helping me.
I have ME/CFS 25 years now but only received diagnosis aprox 3 years ago. I tried specialist services in the UK first but gave up when they prescribed GET in April 2021 as it made me worse.
This made me look about for other possible avenues as I couldn’t carry on as I was.
I have noticed oxidative stress comes up a lot so have concentrated the last 18/12 months on trying to reduce this. I take aprox 1000mg Vit C per day spread out across the day. A good multi Vitamin and Mineral supplement, Dr Myhill’s minerals for sensitive stomachs, transdermal Vitamin B12, Veg epa capsules( 2 per day) (these contain Omega fatty acids and vitamin E plus evening primrose oil). I also take probiotics and Phyto V which includes curcumin, pomegranate, resveratrol, citrus sinensis fruit, and camomile. Some of these are mentioned above by Nick who consulted Chatgpt as being possibly helpful.I also use epsom salts for baths.
I can now look back on how I was feeling a year ago and see good improvements, I am not saying recovery at all, but my dizziness and coordination have both improved, my general malaise ( viral type pain and aching feeling particularly in my neck and shoulders) has also improved. My energy must have improved a little as I can now manage a bit more than I was and my heart rate has improved, not going so high and more quickly coming back to normal).
I know other people have tried supplements and have had no benefit but these or at least some of them have been beneficial to me.
Best of luck with your health journey.
RT
what about dr myhill
she has a protocol b12 vit /folate
and says that there is a blokkade of mitochondrien
what must we believe about blokade of mitochondrien
Dear Cort,
You made what could be a very complicated story very readable and… EXCITING.
Thank you!
Jason
Frequency e.g. any idea what % of people with ME/CFS this affects?
Possibly the (UK) DecodeME (genetic) study will provide some clues re the frequency in the ME/CFS population.
Here’s an article on this same story from the Washington Post:
https://www.washingtonpost.com/health/2023/09/17/fatigue-cfs-longcovid-mitochondria/
I have the mutation for LI-FRAUMENI & show the WAS Gene for Wilkott-Adrich Syndrome.
Does anyone know what medicine Dr. Hwang used on the woman? thank you all. I see my Doctor next Wednesday