

It would seem remarkable if the muscles were not affected in ME/CFS – and indeed French and Italian researchers have been focusing on them.
The muscles. Is there any tissue more likely to be affected in such an exertionally challenged disease?
We just saw NIH researchers undercover a possible mitochondrial abnormality in the muscles of people with chronic fatigue syndrome (ME/CFS). With rather impeccable timing, Yves Jammes – one of the few ME/CFS muscle researchers – is back! A professor of physiology at Aix-Marseille in France, Jammes has managed to publish 8 studies mostly on the muscles in chronic fatigue syndrome (ME/CFS) since 2005.
Over in Italy, Stephania Fulle has been exploring muscle issues in ME/CFS for over 20 years. In the very small muscle studies section of ME/CFS research, they’ve been a ray of light in the darkness.
It’s time to do a little catch-up.
Two Different Types of Fatigue
Two different types of fatigue – central and peripheral fatigue – have been illuminated. It might not be surprising to anyone with ME/CFS that both have been found in this disease.
Central fatigue is produced by the brain and refers to the inability of the brain to get signals to the muscles to work. At least four studies have found evidence that central fatigue is present in ME/CFS. Both kinds of muscle failure – peripheral and central fatigue – have been found in ME/CFS.
“Peripheral muscle failure” is produced in the body. It can result if the muscles aren’t getting enough oxygen, if the muscles aren’t responding to nervous system signals, and/or if a damaged muscle membrane is present. Peripheral muscle fatigue is often closely linked to high levels of free radicals or reactive oxidative species. At least 4 studies have found evidence that peripheral fatigue is present in ME/CFS.
This blog is focused on peripherally produced fatigue.
Damaged Muscle Cell Membranes
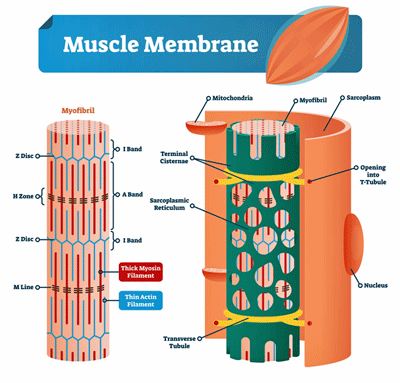
Evidence of muscle membrane damage has been accumulating in ME/CFS for decades.
Fulle and Jammes have been focusing on potential problems in muscle cell membranes in ME/CFS for almost 20 years. They’ve often analyzed the M-waves produced by the muscles as they contract and return to form during exercise.
The M-wave analyses Fulle and Jammes typically use assess how well the muscles are being activated during a fatiguing exercise. Reduced M-wave activity is typically associated with problems with damage to the sarcoplasmic reticulum – the membranes that store the calcium ions that enable the muscles to contract.
Stephanie Fulle got the muscle issue in ME/CFS off to a good start in 2000 when she found evidence of oxidative stress- (free radical) induced damage to the DNA and lipids (read muscle cell membranes) in the muscles of people with ME/CFS. That made sense as studies have shown that exercise creates much more oxidative stress in people with ME/CFS. Plus, numerous studies have found increased oxidative stress levels at rest in ME/CFS as well. Whatever else ME/CFS is, it is a disease of increased oxidative stress.
In 2003, Fulle found evidence that the membranes of the sarcoplasmic reticulum – which store the calcium muscles need to react – had been damaged. When calcium levels in the muscle cells rise, they contract. When they move back into the sarcoplasmic reticulum, the muscle cells relax. It’s this contraction-relaxation process that produces muscle activity and force.
She also found problems with both the (Na(+)/K(+) ionic pump involved in muscle cell excitation and muscle cell contraction as well as the Ca(2+)-pump involve in muscle cell contraction in ME/CFS. At a quite fundamental level then, the muscle cells in ME/CFS were not looking good.
Jammes’s small 2005 study started off his long interest in oxidative stress, muscle membrane excitability (m-wave amplitude), and exercise in ME/CFS. His finding – that high rates of oxidative stress were associated with reductions in muscle membrane excitability (the ability of the muscles cells to respond) in ME/CFS led him to conclude these two “objective signs of muscle dysfunction are sufficient to explain muscle pain and post-exertional malaise reported” in ME/CFS.
In 2008, Fulle proposed that “specific critical points in the muscle” were being affected by free radicals (reactive oxygen species) in ME/CFS. Her 2009 study also found that a switch to “fatigue-prone, energetically expensive” muscle fibers had occurred in ME/CFS. (A similar switch has been found in fibromyalgia.)
The first gene expression (or transcriptome) analysis of muscle tissues done in ME/CFS in 2009 suggested that the muscle cell studies were on the right track. Altered genes involved mitochondrial and oxidative stress, energy production, muscle structure, and muscle fiber type.
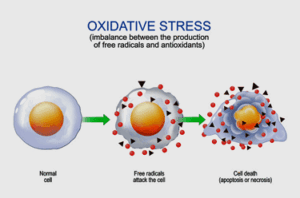
High levels of oxidative stress damaging muscle membranes have been a theme from the beginning.
THE GIST
- The muscles showed up in a big way in the last blog on a potential muscle mitochondrial connection in ME/CFS.
- While one would think that muscles might have been a big deal in such an exertionally challenged disease, they’ve never been front and center.
- Yves Jammes and Sofia Fulle – a French and an Italian researcher – have been studying the muscles in ME/CFS for over 20 years though. With Jammes publishing a recent paper, and with interest in the muscles heating up, it’s time to see what they’ve found.
- They’ve both been focusing on potential problems with the muscle cell membranes. It turns out that the muscle membranes – particularly the sarcoplasmic reticulum – which hold the calcium that the muscles need to contract – are highly susceptible to oxidative stress.
- Studies of electrical fields called M-waves are used in many studies to assess how well the muscles are responding to fatigue, and studies show reduced M-wave activity in ME/CFS. Studies suggest that the more reduced the M-waves are, the more post-exertional malaise and symptoms ME/CFS patients exhibit, and the less able they are to provide muscle strength. (Note, though that not everyone with ME/CFS has reduced M-waves, but the people who are are sicker).
- Since healthy muscle membranes are essential for proper M-wave activity, the reduced M-wave activity found – even at rest in ME/CFS – has pointed a finger at the muscle membranes.
- Indeed, damaged muscle membranes have been found. High levels of “oxidative stress” (free radicals) are most likely the cause of the membrane damage in ME/CFS and Jammes has found a possible reason why – low levels of the protective heat shock proteins that should be protecting the membranes.
- In fact, high levels of free radicals (or reactive oxygen species) could be producing much in ME/CFS. Paul and Lemle propose that an infection-triggered mitochondrial breakdown in long COVID and ME/CFS disrupts the redox (oxidative stress) balance, and produces massive levels of free radicals, which then feed an inflammatory process that impacts the blood vessels, the brain, the muscles, etc.
- Indeed, while Jammes and Fulle have been pointing a finger at the muscle membranes for two decades now, recent research has been focusing more and more on damage to the membranes that surround our cells.
- With 2 major Open Medicine Foundation-funded muscle studies, as well as further NIH muscle work, and a Solve M.E. Ramsay muscle study underway, the muscles are finally becoming something of a hot topic in ME/CFS research. We will hopefully learn much more about them in the near future.
By 2009, Jammes was asking himself why oxidative stress was having such an effect on the muscle membranes in ME/CFS. His very small 2009 study suggested that low levels of the heat shock proteins that protect the membranes were present. Plus, he provided more evidence that damaged muscle cell membranes were present in ME/CFS as well. His much larger 2012 study (73 patients) validated his 2009 results.
In 2020, Jammes proposed something radical. While exercise – which produces lots of oxidative stress – might be expected to damage the cellular membranes in ME/CFS – being in a restful state certainly shouldn’t. Jammes found, though, altered M-wave activity not just in exercising muscle in ME/CFS but in resting muscle as well.
Plus, his study provided evidence that oxidative stress levels were indeed associated with the ability of the ME/CFS patients’ muscles to respond to exercise. Both before and after exercise the muscles of ME/CFS patients with higher oxidative stress levels responded more poorly.
It was not surprising then, to see Jammes propose that high oxidative stress levels were causing a “systemic disorder of muscle membrane excitability” in ME/CFS. As Fulle had earlier, he proposed that the crucial Na+–K+ pump that regulates the muscle contraction process was failing. (These pumps are found in cells across the body and are susceptible to oxidative stress. Jammes asserted that this “strongly suggests that sarcolemma fatigue (read muscle membrane problems) is responsible for the post-exercise muscle force failure” in ME/CFS.
Jammes’s 2023 paper, “Consequences of sarcolemma fatigue on maximal muscle strength production in patients with myalgic encephalomyelitis/chronic fatigue syndrome“, asked if the reduced M-wave (reduced muscle activity) activity resulted in reduced muscle strength in ME/CFS as well. In a prior study, Jammes showed that reduced handgrip strength in ME/CFS was associated with a reduction in maximum energy production during exercise.
In this study, two groups of patients were found – one with normal EMG readings and one with abnormal EMG readings. People with abnormal M-wave readings had more symptoms and more post-exertional malaise after the exercise compared to the ME/CFS patients with normal M-wave activity.
Plus, reduced handgrip strength was associated with reduced M-wave activity; i.e. with reduced activation of ME/CFS patients’ muscles in a large subset of patients. With this new study, Jammes was able to potentially link a reduction in the ME/CFS patients’ ability to produce force with a reduced ability to activate the muscles. Both Fulle and Jammes believe that damage to the muscle membranes is caused by increased levels of reactive oxygen species (ROS); i.e. free radicals.
Oxidative Stress and Membrane Damage
High levels of oxidative stress are perhaps the most consistent finding in ME/CFS. Every study that I know of that has looked for it has found both increased levels of oxidative stress and reduced levels of antioxidants. They are particularly interesting because the mitochondria produce so many of them and damaged mitochondria can produce even more.
Paul and Lemle, for instance, propose that an infection-triggered mitochondrial breakdown in long COVID and ME/CFS disrupts the redox (oxidative stress) balance and produces massive levels of free radicals, which then feed an inflammatory process that impacts the blood vessels in particular, but also the brain, the muscles, etc.
Over time, in diseases like ME/CFS with reduced antioxidant levels, they believe that a positive feedback loop is established: the high levels of mitochondrial-produced reactive oxygen species (oxygen-based free radicals) damage the endothelial cells lining the blood vessels – producing inflammation – which produces more free radicals – which causes more damage, etc. Essentially a fire gets lit that never gets put out.
Membrane Damage (Again)
Cell membranes are like catnip to oxidative stress or free radicals. In their attempt to rebalance their energy state, free radicals love to rip holes in cellular membranes. High levels of oxidative stress go hand in hand with cellular membrane damage.
The membranes that cover our cells protect them from pathogens, toxins, and free radicals and allow them to communicate with other cells, presenting the conduit through which all signals to the cells must pass. If the signal can’t get through to the cell, it can’t react to anything properly. It might as well be inert.
Recent ME/CFS studies have raised the question of whether serious damage to the cell membranes has occurred in ME/CFS. Fulle and Jammes have been raising the same question with regard to the membranes surrounding the muscles in ME/CFS for about 20 years.
Perhaps because Fulle and Jammes are lone wolves working in countries without a history of much ME/CFS research, their studies don’t seem to have received much attention.
With their 2021 hypothesis, “Pathophysiology of skeletal muscle disturbances in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS),“ Wirth and Scheibenbogen, though, made ionic muscle pump problems a central part of their hypothesis which added B2AdR receptors, the sodium-calcium exchanger and the sodium proton exchanger to the mix.
On the long COVID side, muscle-mitochondrial problems have already shown up.
An Explosion of Muscle Studies
Fulle and Jammes are getting some help. The Open Medicine Foundation leads the pack with two major muscle studies from David Systrom and Wenzhong Xiao.
One consists of a deep, deep dive (genomics, proteomics, metabolomics, phospho-proteomics, ultrastructural analysis, mitobiogenetic markers) into muscle samples from ME/CFS patients.
The next will take muscle samples before and after a two-day CPET exercise test and, among other things, assess levels of citrate synthase (which Systrom has found depleted in ME/CFS before), gene expression, metabolites and proteins in the muscles- – and mitochondrial functioning, cytokine, gene expression, metabolites and proteins in the blood.
- Interested in participating in an Open Medicine Foundation study? Sign up here.
Paul Hwang of NHLBI and Avindra Nath are continuing to collaborate on their muscle cell findings and Rob Wust’s Solve M.E. Ramsay award is examining muscle biopsies before and after exercise.
Conclusion
Through a series of sometimes small studies, Jammes and Fulle have put together a nice picture of neuromuscular dysfunction in ME/CFS driven by high levels of oxidative stress. Not only have they found direct evidence of damaged membranes (as well as DNA), but they’ve been able to show that people with reduced muscle activity – thought to be the result of damaged membranes – experience more PEM, have more symptoms, and can produce less force.
Jammes’s studies suggest that the heat shock proteins that protect muscle cells as they become fatigued are reduced. Fulle’s muscle tissue gene expression study highlighted a slew of genes involved in the usual suspects – energy production, mitochondria, oxidative stress, etc. – that were altered in ME/CFS. Plus, her study suggests that the muscle tissues have been altered, and more fast-twitch, quick-fatiguing muscle fibers are present.
Because about half of the patients had reduced muscle excitability, while the other half did not, that finding cannot explain all the exercise intolerance or the PEM that’s present in everyone with ME/CFS.
The Open Medicine Foundations’ deep dive into muscle cells and exercise, the NIH’s continuing research into ME/CFS muscles, and the recent Ramsay Award should tell us much about this potentially critical area of ME/CFS research.






So do free radical scavengers like Vitamin C, E, glutathione or carotenoids help with this? Do they get into muscle? And would IV versions of them work better than oral?
It may not surprise you that glutathione is considered to be a ME/CFS therapy by some functional medicine doctors and that one small study suggested E improves cardiovascular function/ reduces chest pain in ME/CFS patients….
For sure glutathione can help some people. If you check out the Bindu Paul and Marian Lemle blog (linked in the blog) they’re looking for more effective antioxidants (cysteamine is mentioned) that support hydrogen sulfide.
Limited brain right now so I’ll have to come back for a full read, but I just wanted to point out that one function of the sarcoplasmic reticulum is to store acetylcholine. You know, the stuff Mestinon tries to keep around for longer.
Has anyone tried acetylcholine?
Very interesting, thank you Cort! 🙂
Maybe add the meaning of EMG abbreviation to the text?
I agree it likely does not explain all of PEM. I can sometimes feel a metabolic “tipping point” during onset of a crash that feels more like it happens somewhere central.
So would this relate to Ron Davis’ “nanoneedle” paper findings, too (with regard to ionic pump)? As far as I remember, Scheibenbogen / Wirth’s paper referenced it.
Have a nice day (and I hope you’re comfy in the new trailer!)
I don’t suppose acetylcholine is directly available as a medication (apart from application to the eyes)? I might try Cholin. I have not yet tried Mestinon. I think there is a supplement called Huperzine A that does the same as Mestinon. Both are also mysthenia gravis treatment drugs. I am interested in trying because someone with LongCovid mentioned it made a difference. I am confused though because I use anti-cholinergic drugs like trimipramine to calm nervous arousal, so I’m unsure if amping up acetylcholine would really help.
JR, you know the autonomic nervous system is generally divided into at least two parts, yes? ACH runs the central (in brainstem) side of the parasympathetic (rest and digest) system and the peripheral (at muscle) side of the sympathetic system (fight or flight) so it could be that in different areas it has different effects. But I thought trimipramine targeted norepinephrine not ACH so maybe it’s that it’s a different target?
Thank you, Kira! About Trimipramine, as far as I know, tricyclic antidepressants (TCAs) like Trimipramine are an older drug class that has a broader range of effects including anticholinergic effects. In addition my Dr. explained there can be a difference which effects prevail depending on low dose or normal/high dose. (For example at low dose dampening effects of central nervous system prevail, while antidepressant effects set in at higher dose). I use it low dose for sleep in combination with melatonine. Wiki says that compared to other tricyclic antidepressants Trimipramine lacks norepinephrine reuptake inhibition so I don’t suppose it targets norepinephrine, but other TCAs do.
Good to know, thanks 🙂
Acetylcholine breaks down quickly and may not survive the stomach, so generally the best thing to do is to take a drug that prevents its breakdown. (There’s a molecule called acetylcholinesterase that exists in the body to disassemble it.) there are acetycholinesterase inhibitors – including Mestinon!
I just recently started Mestinon and it has been an absolute game changer, helping to reverse a stubborn crash that had persisted for eight months. Another patient says “you will pry it from my cold dead hands before I give it up” and that’s been my experience also.
Oh wow, that’s great to hear, congratulations :-)! At what dose to you apply it for ME/CFS?
One gradually escalates the dose. I was v happy at 90mg/day and am having trouble adjusting to 120 which is my target dose – I’m giving it a week to see if I stabilize, and if still not happy, I’ll go back down to 90 and stay there.
Thank you, have filed away for future reference 🙂 Before that, up next for me in a few weeks is @TheNicotineTest (experimental nicotine patch treatment).
Everything discovered and well-explained here by Cort hits all my lived experience. As a long-time athlete (running, water- and snow-skiing, horse training and riding, etc.) who has had 5 orthopedic surgeries, I can say there has been a radical difference in how my muscles responded during rehab for my 3 pre-ME/CFS surgeries compared to my 2 post-ME/CFS surgeries. It has been the devil to try to explain to a therapist who has seen me for both pre- and post- ME/CFS rehabs how very different things are. [Distinct but not separate from the issue of wanting to avoid a major ME/CFS crash after surgery]. Now, how to fix it? Hoping against hope.
Very interesting Cort. I think you ‘forget’ another small study from professor Newton 🙂 Wasn’t the NO/ONOO-Vicious Cycle Mechanism from dr.Martin Pall? Inflamation is the key in ME/CFS, i think.
(…)the authors examined cultures of isolated skeletal muscle cells (obtained by needle biopsy of the vastus lateralis muscle) from 10 people with ME/CFS and 7 age-matched controls. Electrical pulse stimulation (EPS) was applied for up to 24h to simulate an ‘exercise challenge’ by inducing contraction in the cultured myotubes, so that the effect of ‘exercise’ directly on the cells themselves could be observed(…)
https://www.meresearch.org.uk/muscle-cell-abnormalities/
Very interesting read if a little over my head 🤯 😆
When I was living in Saudi Arabia in 2012 I saw a doctor and told him I suspected I had ME . He then replied he’d need to do a muscle biopsy to confirm it. I thought he was a bit mad at the time so didn’t pursue his suggestion. Wish I had now after reading this article. At that stage I could no longer exercise. I kept trying but the exhaustion, the PEM was too much. At the weekend I had to go downstairs twice (to let the dog out 🙄. Hubby did two trips too and didn’t want to wake him ) and on my second return I had to stop four times whilst climbing and rest on the landing for 10 minutes before completing the rest. It reminded me of Jen Brae in her film how she crawled along the floor and stairs. First time I’ve felt quite scared. Am 99% bed bound.
I wonder what the cure would be for such findings?
Could anyone explain oxidative stress more simply please? 😬🙈
I am really curious as to how these findings compare with what we know about fibromyalgia muscle issues (after all, fibromyalgia I would say is more defined by muscle pathology in some ways than ME/CFS?) I know there is some overlap but don’t know if all the same parameters have been looked at in studies.
Lots more muscle research in FM! Need to do a another blog on that.
Appreciate this important reporting, Cort – and the shoutout! 🙂 Really hoping further findings in this muscle investigation can move us forward.
(quick side note) I saw in Mass ME’s August newsletter: “Congratulations to ME/CFS Advocates in Minnesota! A group of dedicated advocates helped to craft legislation that establishes a comprehensive, statewide monitoring and support program for individuals suffering from Long COVID, ME/CFS, Dysautonomia, and POTS. The legislation is included in the state’s 2023 Omnibus Health Care bill, signed by the Governor and implemented as SF2995, Section 50: Long COVID and Related Conditions Assessment Monitoring. The legislation is supported by funding for the Minnesota Department of Health in the order of $3.14M per year for each of the next 4 years.”
This feels like a pretty big advocacy win for the community – will there be future reporting on this? Just was curious.
Thanks so much for this Cort, it makes a lot of sense. I’ve always said that, for me anyway, ME is as much about muscle weakness as it is about fatigue. And toxicity too.
Just a little word of warning for anyone thinking of trying glutathione. It can set off an allergic reaction. I got an itchy rash from taking it, although I did find it helpful.
As always, thank you Cort for doing such a great job! I am one of the ME/CFS patients inflicted with the muscle issues (burning, weakness, tingling, can’t go up or down any steps or any incline). Neurologist has done all of the standard testing including a muscle biopsy of which he didn’t know how to interpret the pathology findings so he told me to ask my CFS doctor. I actually had an appt with Dr. Systrom a couple of months ago after a long 1.5yr wait and 26 hr round trip drive. I brought the muscle biopsy findings, described all my severe muscle/leg symptoms and his response was “I’m not a Neurologist”…end of conversation. (I will leave out my commentary on the amount of disappointment I felt) I only share this to say, I am beyond hopeful that something will come from these studies but at the same time I am perplexed that he is one conducting a muscle study; yet, had no response at all when I brought up the subject. My biopsy even describes sarcolemmal and endothelial issues causing myofiber damage and myofiber degeneration.
Ouch! That must have been disappointing! The only thing that I can think is that Systrom’s muscle studies are focused on things like mitochondrial activity, proteomics, metabolomics, gene expression and stuff like that while it sounds like yours – as most would be I imagine – might have bene more focused on structural issues (???).
I guess the question now is how to find someone – a different neurologist? – to interpret these findings?
With the sarcolemmal damage to the membrane covering the muscle cells it sure seems like you fit the profile in this study. I see that both the Mayo and Cleveland Clinics have neuromuscular centers.
Jill, you might try a neuromuscular specialist. I saw one recently out of Columbia in NYC – her name was Dr. Comana Cioroiu, or you might seek out one nearer to you.
I am mystified about why any doctor would prescribe PB (Mestinon) pills for ME/CFS patients.
Our organization has worked with Gulf War veterans since 1990. Nearly 34% of veterans who served in the first Gulf War have Gulf War Illness which is almost identical to ME/CFS, any differences may be due to the preponderance of GW Illnesses in males and ME/CFS in females.
This is an excerpt of a study funded by the Veterans Administration reporting on the use of PB pills by the veterans.
“Both PB pills and pesticides exposures have been linked to other aspects of GWI in prior studies. A study of 604 GW veterans examined relationships between neurotoxin exposure and four GWI domains (neurocognitive/mood, gastrointestinal, fatigue/sleep, and pain) [10]. Exposure to both pesticides (OR = 4.13, 95% CI = 1.78–9.57) and PB pills (OR = 2.28, 95% CI = 1.02–5.09) were strongly associated with pain severity. Pesticides were also associated with difficulties in concentrating (OR = 2.29, 95% CI = 1.04–5.02) and recall (OR = 2.18, 95% CI = 1.04–4.56), difficulty with sleep (OR = 3.06, 95% CI = 1.19–7.89), unrefreshing sleep (OR = 3.13, 95% CI = 1.09–8.98), and joint pain (OR = 2.46, 95% CI = 1.04–5.82). PB pill use was associated with depression (OR = 2.68, 95% CI = 1.28–5.60) [10]. In our study, we did not examine sleep quality but did find that individuals exposed to PB and pesticides were significantly more likely to be diagnosed with sleep apnea compared to their respective controls. This association has also been described in the setting of pesticide use outside military exposure. One prospective study examined 1569 pesticide applicators in the US farming community who reported exposure to any one or more of 63 pesticides [38]. Of those pesticides, an association was noted between sleep apnea and carbofuran, a carbamate pesticide (OR = 1.83, p = 0.0002) [38]. This suggests that exposures during the GW arena may impact the frequency of co-morbidities beyond the eye, including sleep and mental health.”
I tell people that I have CFS and Fibromyalgia (although these “best fitting” labels only describe symptom clusters, and not the cause(s) of symptoms). In any case, for what it is worth, I still have good muscle strength. I am still able to carry two 50 lb water bottles from the car to the house, and I can still beat my doctor in an arm wrestle (even though he is slightly younger and stockier than I am). However, a walk around the block can bring me close to collapse. Thus, my personal energy problems have little to do with strength, but a lot to do with stamina.
I found this article very interesting. I have parkinson’s disease : in 2012, I was diagnosed with hyperparathyroidism requiring surgery to identify the bad gland (there are 4). Hyperparathyroidism causes. calcium which is needed for proper muscle, brain and other critical functions. to be pulled into the bloodstream., thereby leaving insufficient calcium in the body where it needs to be.
I had to figure this out myself with 2clues : 1) remembering that my most recent blood test showed a higher than normal blood calcium level: and a good radiologist who wrote in a report for a thyroid ultrasound that she “thought” she might be seeing parathyroid abnormality. I found parathyroid specialists in Florida that.specialized only in parathyroid surgery. For a reasonable fee, they evaluated my relevant records. I also.had osteoporosis because calcium was leeching out of places where calcium should be eg. muscles.into bloodstream. My muscles are and we’re very weak, now all complicated with. Parkinson’s disease. The osteoporosis was reversed to osteopenia within 2 years post op. I was told.that in adults 35 or older should not have calcium in the blood over in the 9s. Mine was over 10.5. Many doctors and endocrinologists do not know about these more unusual conditions, leaving hypercalcemia undiagnosed and left to do damage to brain, muscles, bones and teeth. etc.
Hey Cort, I am bit shocked that you didn’t mention the direct link between this study and Dr Tremblay’s.
I am talking about Sarcoplasmic reticulum which is made of plasmalogen !
Oh my! Look at that. That’s pretty exciting!
(I can only hope that it came out after I did the blog :))
https://pubmed.ncbi.nlm.nih.gov/37423295/
Thanks for the tip 🙂
There are studies that show that muscle fiber % in chronic fatigue is significantly shitfted towards fast-twitch fatigueable fibers.