

“AI has literally changed my life, being an ME/CFS patient for many years and now symptom-free since 2015. AI has identified well before several findings that have been identified at a later time by conventional research”. Efthymios Kalafatis

A data scientist Efthymios used artificial intelligence to try to get at core aspects of ME/cFS.
Efthymios’s Background
After his studies in computer science Efthymios Kalafatis got an MSc in knowledge extraction. He’s been working since 2000 in data mining and later machine learning and natural language processing consulting. He has a 2018 patent named “Machine Learning, Natural Language Processing, And Network Analysis-guided Discovery Related To Medical Research“.
In 2022 he was a co-author of a paper examining LongCOVID in Greece and is the principal investigator of a Ramsay Award grant from the Solve ME/CFS Initiative that will use data from the You+ME Patient Registry to identify symptom, treatment, activity, and life event patterns in ME/CFS and long COVID.
His onset of ME/CFS in 2004 began a long decline in his health. In 2012 he used machine learning and other tools to associate his personal factors (food, supplements, activity, etc.) with his good days and bad days. The lessons he learned from that experiment allowed him to stabilize his health.
In 2013, he began using artificial intelligence methods to attempt to make some headway in the ME/CFS. In part because ME/CFS is under-studied, his idea was to capitalize on the massive amount of data concerning diseases closely related to ME/CFS in order to learn more about ME/CFS.
To that end, he started by collecting via software the abstracts of all the published research regarding the symptoms found in ME/CFS such as fatigue, orthostatic intolerance, depression, anxiety, tinnitus, etc. He also collected all published research related to ME/CFS, fibromyalgia, Gulf War illness, post-treatment Lyme disease syndrome, post-finasteride syndrome, and post-Accutane Syndrome. (Efthymios chose those diseases because he read about many cases of patients of these syndromes ending up having ME/CFS).

Efthymios used AI’s ability to plow through enormous amounts of data to uncover biological hotspots in ME/CFS and related diseases.
The next step was to identify -via text analytics- the most frequently occurring potential biological factors such as genes, biological pathways (e.g. mevalonate pathway), and other biological entities (e.g. mitochondria) that existed within these abstracts but also adding medical concepts as these were identified to be important year after year by researchers.
That list of concepts prompted another search that resulted in published research for each of these topics. That resulted in millions of scientific abstracts being collected which resulted in the next text analysis finding associations between genes, biological pathways, and symptoms.
The first analysis involved a type of machine learning analysis called classification analysis during which all relevant symptoms were grouped. Machine learning algorithms were then used to identify potential key biological factors associated with those symptoms.
By 2014 the first AI results were out: they identified endoplasmic reticulum (ER) stress, oxidative stress and many liver-associated factors as important targets. The results were ahead of their time as ER stress was just recently identified by NIH researchers as a potentially important contributor to ME/CFS (https://pubmed.ncbi.nlm.nih.gov/37579159/).
Using the results from the artificial intelligence study Efthymios was able to, after 11 years, regain his health. See his recovery story here.
At the end of 2015, Efthymios sent an email to ME/CFS researchers proposing that liver injury (that does not always show up in tests) in conjunction with genetic polymorphisms (slight changes in genes) involved in a variety of areas (cytochrome P450 pathway, methylation, glucuronidation, choline metabolism, bile acid synthesis and enterohepatic circulation, oxidative stress and endoplasmic reticulum stress) resulted in uncontrolled endoplasmic reticulum and oxidative stress and the unfolded protein response.
A new development in artificial intelligence in 2015 that allowed any possible biological pathway, gene, or compound to be given a relevance score enabled Efthymios to take the next step. Now AI programs are better able to pluck out the key factors in a disease.
In 2017, Efthymios added an additional analytical method named “Network Analysis” which researchers such as Nancy Klimas and Derya Unutmaz have used in ME/CFS. His goal was to create a knowledge map that highlighted prominent factors in ME/CFS. He succeeded. His findings (which have not been published) are below.
Hotspots Identified
The tightly correlated findings with their clear center that resulted (see below) suggested he was on the right track. Contrary to those who believe ME/CFS is a heterogeneous mess, Efthymios’ analysis suggested that core areas of high biological interest – or hotspots exist. (Red denotes highly important topics, orange less important, blue lower importance).
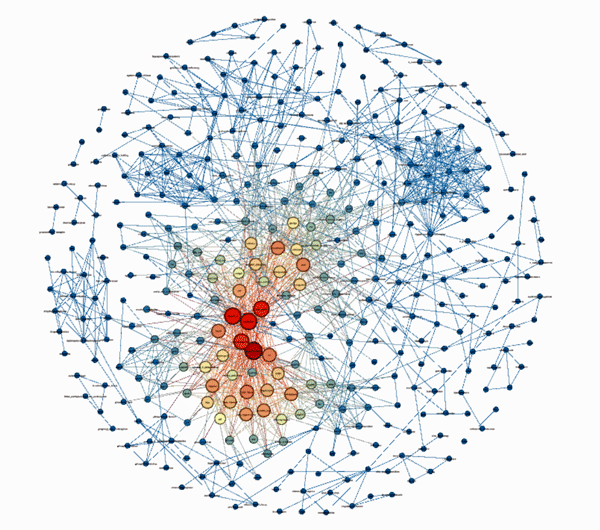
Look at the concentration!
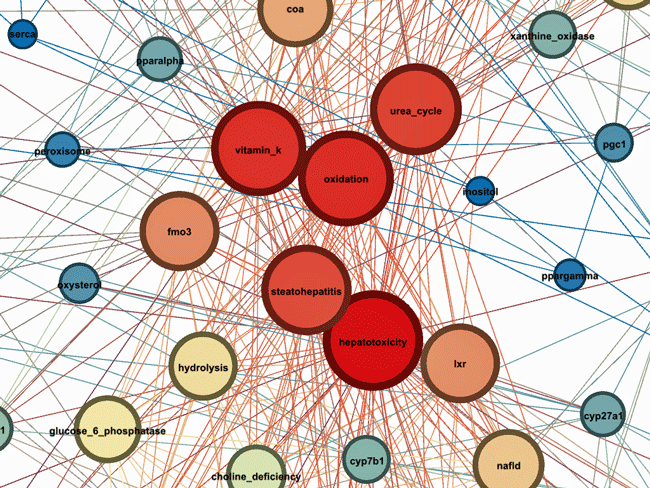
Some hotspots
The Liver Shows Up Big Time
Of the five “hottest” or reddest areas two (hepatotoxicity -liver damage), steatohepatitis (fatty liver disease) involved the liver. The others involved oxidative stress – long known to be present in ME/CFS, vitamin K (blood coagulation, calcium metabolism), the urea cycle (ammonia – a possibly big player in ME/CFS), fmo3 – a liver enzyme which can be found in the endoplasmic reticulum and is responsible for the metabolism of several xenobiotics, and LXR (a liver receptor) which has been recently (August 2023) identified in a paper by Maureen Hanson’s group and was found downregulated in COVID19.
These results suggested that liver injury caused by viral infections, medications, or other environmental factors provides a link between syndromes like ME/CFS, fibromyalgia, Gulf War Illness, post-treatment Lyme disease syndrome, etc. It also proposed that concepts related to oxidative stress, the liver, bile acids (CYP7B1, CYP27A1), peroxisomal function, mitochondrial biogenesis, detoxification, phospholipid metabolism (choline_deficiency) had to be looked at.
I was surprised at how often the liver showed up and asked Efthymios about it. He said “I did not know what to expect really. I was surprised though that it was showing over and over again as new information was being fed into the AI system as years passed by. And I believe some other researchers may now be getting similar signals (note that normal liver enzymes do not rule out liver disease!)”
A Liver ME/CFS Connection
It turns out that the liver has shown up quite a bit recently in both ME/CFS and long COVID. The urea cycle which converts ammonia to urea in the liver cells, in particular the mitochondria of liver cells, has shown up in at least three metabolomic studies including two by the Hanson group as well as a gene expression study.
Germain’s 2018 “Metabolic profiling of a myalgic encephalomyelitis/chronic fatigue syndrome discovery cohort reveals disturbances in fatty acid and lipid metabolism” study found reductions in primary bile acids that were suggestive of liver damage. Plus the pathway with the highest “impact factor” was taurine – which is part of the primary bile acid biosynthesis pathway. The authors noted that lower levels of taurine – which was part of Efthymios’s recovery protocol – have been found in ME/CFS and that the supplement is used by many ME/CFS patients. Plus one of the most impactful pathways found – the glycerophospholipid metabolism – is dependent upon proper liver functioning.
The authors even went so far as to suggest that a panel for hepatotoxicity prediction might constitute a serum metabolic signature for ME/CFS. (The paper did note that the reductions could reflect toxicity from the use of prescription or over-the-counter drugs (high doses of acetaminophen/Tylenol can impact the liver)
In April 2022 the Morten Group at Oxford asked “Could an upset liver be a key clue in ME/CFS?” and stated their metabolic screen “found a consistent liver issue in mild, mod, and severe patients”. Chris Armstrong of the Open Medicine Foundation is currently engaged in a project to understand what’s going on with ammonia metabolism in ME/CFS. It turns out that when a dysfunctional liver fails to break down nitrogen, excess ammonia can produce neuroinflammation and encephalopathy. It was the attempt to understand what’s happening with ammonia that led to the itaconate shunt hypothesis. (A blog on the hypothesis is coming up.)
A Liver Long COVID Connection
With regard to long COVID a presentation at the Keystone International Long COVID conference proposed that liver dysregulation in long COVID may be, among other things, whacking the mitochondria. A recent conference report also found increased levels of “liver stiffness” months after the infection. The lead researcher Dr Firouzeh Heidari at Massachusetts General Hospital, said “Our study is part of emerging evidence that Covid-19 infection may lead to liver injury that lasts well after the acute illness.”
Endoplasmic Reticulum Stress and the Unfolded Protein Response
Efthymios’s model also highlighted endoplasmic reticulum (ER) stress and problems with the unfolded protein response (UPR). Proteins are folded into their correct shapes (shape is everything with proteins) in the endoplasmic reticulum (ER). Problems with the ER can cause proteins such as amyloids to gum up the works in the cell. An accumulation of misfolded proteins triggers the “unfolded protein response (UPR)” which then should degrade them. It can also trigger “autophagy” a process whereby the cell uses “autophagosomes” to digest the strangely shaped proteins.
Cells exposed to stressors like viral infections often trigger the unfolded protein response (UPR) and autophagy. Two studies, thus far suggest that problems cleaning up these misfolded proteins may exist in ME/CFS. Baraniuk’s 2005 paper found evidence of amyloid proteins in the cerebral spinal fluid of ME/CFS patients and evidence of impaired autophagy recently showed up in ME/CFS.
The recent WASF3 study highlighted the role that damaged endoplasmic reticulum may play in ME/CFS. Time will tell what role ER stress and the unfolded protein response plays in ME/CFS but they are another example of new findings (like microclots) that potentially open a wide variety of new treatment approaches.
Brian Vastag reported that the lead author is trying to get a clinical trial of an amyotrophic lateral sclerosis drug, Relyvrio, underway in ME/CFS and many different drugs (Dextromethorphan, Bromocriptine, Dantrolene, Verapamil, diltiazem, thalidomide, lenalidomide, pomalidomide, tamoxifen) can impact it.
Xanthine Oxidase
Xanthine oxidase was another hotspot that popped up in Efthymios’s AI analysis. Xanthine oxidase, an enzyme, catalyzes the oxidation of hypoxanthine to xanthine. In his 2019 ME/CFS metabolomic study, McGregor found that hypoxanthine was the principal metabolite associated with post-exertional malaise. McGregor believed that the hypoxanthine increase was associated with the inhibition of protein synthesis in the muscles during exertion. His study suggested that ME/CFS patients’ cells were breaking down amino acids – a poor energy source – to fuel their mitochondria.
AI-Driven Approach Predicts Future Findings
Only time will tell what role the liver, the endoplasmic reticulum, etc. play in this disease but it is notable that Efthymios’s artificial intelligence/network analysis approach was able to pluck out potentially key themes (liver, endoplasmic stress, peroxisomal functioning, phospholipid metabolism, and xanthine oxidase metabolism) which showed up, in some cases, in ME/CFS study results years later.
Note that the clues were already there – buried in research papers on ME/CFS and allied diseases. They simply needed to be unearthed using artificial intelligence methods.
Note that Efthymios’ project took him up to 2017; i.e. six years of data have been produced since then. One wonders what new clues a 2023 AI project might produce given what we’ve learned so far, the emergence of long COVID, and AI’s apparently rapidly accelerating capabilities.
THE GIST
- About ten years ago, Efthymios Kalafatis, a data scientist, used artificial intelligence (AI) to plow through millions of documents and pluck out potentially key hotspots in ME/CFS and related diseases.
- He also developed a treatment protocol that allowed him to recover from ME/CFS. Efthymios stated “AI has literally changed my life, being an ME/CFS patient for many years and now symptom-free since 2015.”
- As AI methods developed over time Efthymios first used machine learning and then network analysis to sharpen his results. The results suggested that instead of being heterogenous illnesses that core hotspots existed not just in ME/CFS but in allied diseases as well.
- The liver, endoplasmic reticulum stress, the misfolded protein response, peroxisomal dysfunction, lipid issues were all highlighted. Most of these were not thought to play a role in ME/CFS at the time but recent studies suggest that all could play a core role.
- The liver, in particular, showed up. Efthymios’s analysis suggested that liver injury caused by viral infections, medications or other environmental factors provided a link between syndromes like ME/CFS, fibromyalgia, Gulf War Illness, post-treatment Lyme disease syndrome, etc.
- Since Efthymios’s analysis (which was never published) several studies have highlighted the liver’s possible role in ME/CFS. In April 2022 the Morten Group at Oxford asked “Could an upset liver be a key clue in ME/CFS?” and stated their metabolic screen “found a consistent liver issue in mild, mod, and severe patients”.
- Recently stressed-out endoplasmic reticulum – the organelles that fold proteins to the correct shape in our cells – were highlighted in an NIH-funded ME/CFS study. Similarly problems with lipid metabolism, the urea cycle and xanthine oxidase have popped up since Efthymios’s analysis in 2017.
- Only time will tell what role the liver, the endoplasmic reticulum, etc. play in this disease but it’s notable that Efthymios’s artificial intelligence/network analysis approach was able to pluck out potentially key themes (liver, endoplasmic stress, peroxisomal functioning, phospholipid metabolism, and xanthine oxidase metabolism) which showed up, in some cases, in ME/CFS study results years later.
- Note that the clues were already there – buried in research papers on ME/CFS and allied diseases. They simply needed to be unearthed using artificial intelligence methods.
- AI is limited in several ways. It can work through huge amounts of data more quickly and accurately than any human can but it cannot explain how it gets the results it does and cannot go beyond the data given it; i.e. it can help explain the past but cannot predict the future.
- Efthymios’s analysis was done in 2017 – six long years ago. One wonders what a 2023 analysis using the data generated since then in ME/CFS, long COVID, and other diseases would uncover and how it might help shorten the search for the answers to ME/CFS and allied diseases.
Next Steps
In 2018 Efthymios presented his AI-assisted research in a talk “Machine Learning and Network Analysis Guided Medical Research” at the Euromene Conference and was granted a patent application “Machine Learning, Natural Language Processing And Network Analysis-guided Discovery Related To Medical Research“.
The goal of natural language processing is to create a program or computer that is capable of fully “understanding” the contents of documents and can “accurately extract information and insights” from them. Being able to feed huge amounts of data from many studies into a computer, find associations and have the computer extract novel insights from them could obviously provide a major step forward in our understanding of disease.
One of Efthymios ‘ goals with his patented methodology is to use AI algorithms and Network Analysis to produce hypotheses on how ME/CFS is related to other diseases. Another goal is to create an ordered/ranked list of high-priority biological topics, symptoms, and environmental, or nutritional factors – which he has done.
It was fascinating stuff and with artificial intelligence suddenly becoming the talk of the town in 2023 I wanted to pick Efthymios’ brain about the possibilities it raises for understanding and finding treatments for ME/CFS and allied diseases. He said :
“AI has literally changed my life, being an ME/CFS patient for many years and now symptom-free since 2015. AI has identified well before several findings that have been identified at a later time by conventional research”.
Questions
How is AI different from, say, the sophisticated statistical programs used to assess complex study results? What makes it different from other data-driven approaches?
“First, some definitions: AI is a technology that attempts to achieve or even exceed the level of human intelligence. Machine Learning (ML) is a subset of AI where the computer “learns” from data given a specific problem (i.e. An algorithm “learns” from the data being presented and automatically identifies subgroups (clusters) of patients according to a number of metabolites).
ML has been heavily relying on statistical methods -no question about that- and there is this huge debate as to whether ML is no different than statistics. In statistics, we have a “tighter” set of rules to analyze and draw inferences from data”
AI has been so in the news lately – 2023 is truly the year that AI made it into the public consciousness. We know that AI can perform complex calculations and analyses faster and more accurately than humans, recognize patterns in large amounts of data that humans would never be able to pick up, etc. I wanted to know, though, what AI cannot do
In something called the “explainability problem,” Efthymios stated that AI is something of a black box in a way. It cannot explain to us why it’s drawing the conclusions it’s drawing. For instance, it cannot say why it asserts that “x” research target should be studied to explain “y” symptom. A technology named XAI (Explainable AI will eventually change this)
AI also cannot go beyond the data it’s given; i.e. if the data on say a gene – is not included in its program – it can never logically infer that manipulating that gene might be helpful. It can only say things about the data it already has.
We’ve been seeing studies using machine learning and other AI techniques for quite some time but this is the year that artificial intelligence showed up big time publicly. Why is that? Has something recently happened to move the field forward in a dramatic way?
I think that Chat-GPT’s widespread use is something to consider here. It showed everyone the immense potential of AI but also that we must proceed with caution”
How are researchers using artificial intelligence right now to understand diseases? Have any breakthroughs been produced?
“Researchers rely more and more on machine learning methods for diagnostic purposes mainly, for example identifying factors that differentiate patients versus controls.
A breakthrough here would be to be able to provide to an AI system like ChatGPT with everything we know about symptoms, metabolites, and any other ME/CFS findings and have it suggest the possible causes (I would happily contribute to implementing this!)
Another breakthrough would be for an AI system to automatically identify patient subtypes and indicate factors as to why specific interventions are extremely helpful to some patients and not helpful to others.”
You stated that software -using non-AI methods- can help us connect various research findings from ME/CFS research within seconds and speed up the knowledge extraction process. This new knowledge could then be forwarded to relevant research teams, enabling them to see the “bigger picture” of ME/CFS and guide resources to the most promising research targets.
How would this work and what would it do? If you were to embark on an AI-driven ME/CFS project right now what would you do differently?

How far could AI go to solving diseases like ME/CFS and long COVID?
“We see many theories and research teams working to identify key mechanisms of ME/CFS. Techniques like Information Retrieval, Natural Language Processing, and Network Analysis can help us identify – with unprecedented speed – which medical concepts may connect these theories with existing findings and other syndromes such as Ehlers-Danlos Syndrome, fibromyalgia, post-treatment Lyme Disease, long COVID, etc.
If I did a project now I would try to capture patient historical data as I believe that important insights may be found in conditions or events that occurred prior to the onset of ME/CFS. LongCOVID would also be included as it appears that many of its symptoms match those of ME/CFS, including post-exertional malaise. Causal AI would also be of interest to try to predict how different interventions change the outcome and why.
“Regarding any hidden connections: Post-Translational modifications -such as Ubiquitination-, apoptotic cell clearance mechanisms, and finally the potential role of CH25H (Cholesterol-25-Hydroxylase) in viral persistence mechanisms should be further investigated according to the methods I have been using”
(Note – “Causal machine learning (CML)… provides a complete toolset for investigating how a system would react to an intervention (e.g.\ outcome given a treatment).”)






Does anyone know Efthymios’s health protocol? I sure would love to know what he did to go into complete remission from ME/CFS.
I was so surprised and disappointed his Protocol wasn’t shared in the article!!
It was kind of obliquely shared – it’s in his recovery story – you can find it here – https://www.healthrising.org/blog/2023/10/02/efthymios-artificial-intelligence-chronic-fatigue-syndrome-recovery/
THANK YOU FOR ADDING THIS LINK!!
Thank you Cort. I’ll have a look!
cort, what is the best place to donate for me/cfs?
Yes, many of us do! 🙂 It’s posted in the recovery story we featured a couple of weeks ago. Its a most interesting protocol. You can find it here – https://www.healthrising.org/blog/2023/10/02/efthymios-artificial-intelligence-chronic-fatigue-syndrome-recovery/
One thing could be in the food anything with folic acid added it in all flour foods gluten, gluten free, additives even in soya food additives the FDA put it in flour back in 1989 there is a genetic mutation in folate & Tulane University on Facebook published this recently thay are on Facebook called Tulane Hypermobility Clinic all the links are there.
It is also in numerous vitamins/minerals folic acid. As many as 51% have EDS issues in the population. His photo to me shows his ear lobes are attached a good sign that one has a connective tissue disorder.
I think he has EDS & no not everyone has these ear lobe issues in EDS, he needs todownload all EDS Papers on AI & not all are bendy. I was told I had EDS 3 & partial Marfans by top Team Hypermobility at Stanmore Orthopedic Hospital in London, then a Genetic Doctor at SGH Princess Anne Hospital in Southampton said I do not have those I do not fit those criteria she said I have HSD so I got ME/CFS with HSD.
I know GSD can go with EDS its not written about but I have asked many groups they say they got both GSD with EDS types. Imagine if 51% have this genetic mutation 23 and me test can show these issues in folate mutation. GSD is Glycogen Storage Disease countless types.
Can we also have (HFI) Hereditary Fructose Intolerance also missed.
His protocol above shows he takes vitamins/minerals with methyl B12 & methyl folate
This statement jumped off the page at me……So true:
“It turns out that when a dysfunctional liver fails to break down nitrogen, excess ammonia can produce neuroinflammation and encephalopathy”.
Epstein Barr virus causes a lot of liver damage.
I have personally experienced vast a quantity of ammonia excretion (via urine) after vigorous activity, as well as migraine headaches and “foggy brain”. When severe my dreams were almost like an acid trip from the ammonia.
Interesting. Chris Armstrong is on the hunt for ammonia in ME/CFS – it’ll be fascinating to see what he finds out.
Yes, I think my liver was affected after I was infected with EBV as a teenager. After more recent viral infections, I have more difficulty processing some foods, especially dark chocolate, foods high in fructose and chemicals in some toothpaste etc. If my liver becomes ‘irritated’, then I feel poisoned, my blood pressure and heart rate increase and my brain is clearly affected. This was picked up when I went into hospital for a procedure and I’d eaten some dark choc a few days previously. A nurse asked me if I’d ever had hepatitis?
And I don’t go anywhere near alcohol. After the glandular fever/mono/EBV infection, my doctor said that I shouldn’t drink alcohol. I didn’t until a few months after I was ill. I took a small bit of an alcoholic drink and felt incredibly unwell and nauseous. So I didn’t drink any alcohol for years after that.
I think I was becoming more intolerant to alcohol before I had a virus in 2007, that appeared to tip me over the edge. I then had massive issues with sugar, carbs and it turned out that I seemed to have a particular problem with processing fructose (which is processed by the liver). High fructose foods, especially corn/maize were the worst. They seemed to induce a sedative effect and would affect my impulse to breathe. I’d have to lie down and would be totally immobile. Not asleep and not fully awake either. I seemed to have stopped breathing one night and woke up in a panic and took the biggest breath, I’ve ever taken. Obviously doctors thought I was just overly anxious, which didn’t help. So, I had to figure it out for myself.
I forgot about the alcohol intolerance connection and didn’t know about the corn/fructose connection. I don’t do well with corn either… Interesting.
This article is by Robert Lustig. He loathes sugar. It’s a fairly old article and there’s probably more current info around but I think it explains the issues.
I couldn’t work out why I didn’t keel over when the doctor did a *glucose* tolerance test on me. Eventually I figured out that I have much more of an issue with fructose. Regular table sugar is half glucose, half *fructose*. Maize, corn and high fructose corn syrup all contain high levels of fructose. Glucose is not the same as fructose.
I didn’t react adversely to the *glucose*, so therefore the doctor thought I didn’t have a real problem.
https://pubmed.ncbi.nlm.nih.gov/23493539/
The corn products points towards pellagra, a B3 deficiency
The high leucine content revs up your metabolism, and if you are hypometabolic and lacking in nutrients, it will further send you into a reduced state.
I grew up on corn prodycts and strangely became intolerant of them for about 12 years until I finally had an acute pellagra attack. High dosed B3 for a while, and now I’m back to being OK with the corn.
There was an outbreak of hepatitis A in my school cafeteria when I was twelve. I became jaundiced and spent a month in the hospital in isolation. As a teenager I had several painful attacks, and I remember vomiting bile. Then, these problems disappeared until my late twenties when a doctor diagnosed gallstones, and I had my gallbladder removed. Within six years of the surgery, I had fibromyalgia, although it wasn’t recognized as a disorder until the late 1980s. I am now 74, and have been dealing with this for over thirty-five years. Supplements have helped with the pain, but my sleep remains terrible. I also now have POTS. I don’t want to take anything addictive so I tried 500mg of Inositol which is a neurotransmitter. It has helped my sleep, and I feel better overall. According to what I have read, it also helps to detoxify the liver and improve liver function, which is interesting considering the results that AI found. It’s been thirty-five years of trying to understand what is going on, and like so many others here, I’m tired of being tired.
So sorry Karen, it’s miserable. I think my liver has been a bit weird since the EBV and seems to be even more reactive now.
Definitely feel like there is a liver connection for me. There are times I am significantly flared up (joint pain, flu-like, fatigue, brain fog) and in the middle of the night I will have a massive night sweat that completely soaks through the sheets. The sweat smells like straight ammonia…literally like a bucket of ammonia was dumped in the bed. Upon waking after such a sweat I typically will feel much better for a while…but it doesn’t last. Usually by the middle of the day I am back to my baseline.
So disappointing not to see the protocol he used shared in this article!
It’s in the link to his recovery story or here: https://www.healthrising.org/blog/2023/10/02/efthymios-artificial-intelligence-chronic-fatigue-syndrome-recovery/
Compromised liver detoxification would also explain Multiple Chemical sensitivity, as well as PEM from exercise metabolites such as ammonia.
I wonder if Efthymios does consultations?
Gail, I do not do any consultations, I am not a medical professional. Currently I am trying to put this technology to use and help identify key aspects of these syndromes, including ME/CFS.
Excellent reporting as usual, Cort!
This is exciting , even given my concerns about AI .
Interesting about the liver. Wonder if any ME peeps have had liver transplants?…. And had subsequent relief from symptoms!
This is very interesting. Based on the treatment protocol in the other article and the information in this one, what would be relevant testing to do? I noticed from the article that normal liver enzymes doesn’t necessarily mean the liver is fine. How and what would you test to know more about the issues discussed here?
Cort, could you find out what Efthymios did, or took as supplements or medicines, that his AI research indicated, & thus cured his ME/CFS?
It’s in his recovery story – https://www.healthrising.org/blog/2023/10/02/efthymios-artificial-intelligence-chronic-fatigue-syndrome-recovery/
In 2018 or 19, tests came back showing I had elevated ammonia. My Primary Care Doctor (who has many ME/CFS patients) explained to me the danger of elevated ammonia for the brain. He put me on the daily supplement L-Ornithine.
This is excellent! It has connected the dots for my sudden onset in 1993. I always suspected that a very high dose of SLO-Niacin which I took right before the onset (hours before ) affected my liver. This was after months of my Doctor ramping it up to lower borderline cholesterol. My tryglecires and cholesterol went crazy the ensuing months. And even during the ramp up they started elevating significantly. My Doctors response was increase the dose.
Note that Ethmios responded to my comment on his previos highlight by court by sharing an article on the hepatoxicity of Slo-Niacin.
Another important piece of info is that tests a few weeks later showed I had high levels of EBV antibodies also. I do not know if that was an opportunistic reactivation or not. But Doctors often have told me that my blood work shows I am recovering from a not too distant EBV infection. I suspect that EBV is involved due it also can affect the liver.
That was 33 years ago. Fast forward to present. One thing that gives me mental and physical energy is Immunocal. A denatured whey protein, but it also kicks in inflammation hard. I have taken it since 2000, even though the inflammation issues. It allows my body and brain to perform at a very high level a few hours a day. Thus I can work from home and support my family with lots of pacing.
I am trying ti replace it with AMINOGLUTAM. My dear Doctor, Morris Papernik recently suggested it. He is a highly respected advocate for PWCs for decades. I ordered it from italy via web. Dr. Papernik said a study found that it helps PWCs. I need to find that study to learn the dose used. I am taking 1/30th the dose recommend in the packed based on how much glutamine I know I can tolerate. If you research AMINOGLUTAM you will find it is used for high oxidative states and the liver. No coincidence that at times it makes me feels normal. But then the effect runs out and I do not feel good at all. I crave more, Yes, this could be because I might need a higher dose. I just need ti find the dose that does not kick in too hard. It also makes sleepy for about an hour after taking it. I learned to be cautious with aminoacid supplements. So It is very important to me to start with very small doses.
Interestingly enough it seems to have an anti inflammatory effect as well.
I wonder what Ethmios thinks about the ingredients.
I have my medical records since before onset. Including labs, liver biopsy reports as well. Maybe these info could help in Ethmios research.
Here is more info on Aminoglutam. Note the key uses and how that may be applicable.
Anyone else tried it?
AMINOGLUTAM SACHETS WHAT IS IT FOR?
Dietary food recommended for the diet of subjects with protein malnutrition (cancer patients or with oxidative metabolic stress, post-surgical or with acute or chronic inflammatory-degenerative diseases), to favor the increase and restoration of muscles (senile or neoplastic sarcopenia ) and tissue repair (surgical wounds, burns, bedsores, etc.).
Aminoglutam in gel sachets improves physical performance and muscle strength, counteracts muscle catabolism and promotes plasma, mitochondrial and muscle protein synthesis. The increase in the number and functionality of mitochondria allows the use of carbohydrate and lipid substrates for a more effective energy production, a reduction of oxidative stress and an enhancement of the immune system. The mechanism is independent of insulin, therefore also active in conditions of insulin resistance related to various pathologies or to oneoplastic senile sarcopenia.
Therapeutic indications of Aminoglutam sachets
Aminoglutams Gel is a dietary food intended for special medical purposes indicated for the diet of subjects with protein malnutrition (cancer patients or with oxidative metabolic stress, post-surgical or with acute or chronic inflammatory-degenerative diseases), to favor the increase and muscle restoration (senile or neoplastic sarcopenia) and tissue repair (surgical wounds, burns, bedsores, etc.).
Ingredients contained in it
Maltodextrin: L-glutamine; L-leucine; thickeners: xanthan gum, hydroxypropylmethylcellulose: L-isine monohydrochloride; L-isoleucine: L-valine; acidity regulator: citric acid; L-threonine; aroma (flavors, inaltodextrins, gum arabic); L-cystine: L-histidine; L-phenylalanine: L-methionine: sweeteners: aspartame, acesulfame K; L-tyrosine; L-tryptophan; L-ascorbic acid; dye: beta-carotene (sucrose, maltodextrin, gum arabic, partially hydrogenated soybean oil, beta-carotene, dl-alpha-tocopherol, sodium ascorbate); thiamine hydrochloride; pyridoxine hydrochloride.
for 2 envelopes for an envelope per 100 g
Power 138 kcal
578 kJ 69 kcal
289 kJ 276 kcal
1156 kJ
Proteins
Carbohydrates 20.4 g 10.2 g 40.8 g
Fat
Nutritious mg / for 2 sachets mg / for one sachet per 100 g
L-glutamine 14000 7000 28 g
L-leucine 3125 1562.5 6250 mg
L-lysine 1624 812 3248 mg
L-isoleucine 1562.5 781.25 3125 mg
L-valine 1562.5 781.25 3125 mg
L-threonine 875 437.5 1750 mg
L-cystine 375 187.5 750 mg
L-histidine 375 187.5 750 mg
L-phenylalanine 250 125 500 mg
L-methionine 125 62.5 250 mg
L-tyrosine 75 37.5 150 mg
L-tryptophan 50 25 100 mg
C vitamin 30 15 60 mg
Vitamin B1 0.3 0.15 0.6 mg
Vitamin B6 0.3 0.15 0.6 mg
How should the product be taken? let’s discover the preparation
Pour the contents of a bag of Aminoglutam Gel into a suitable container and disperse in 80 ml of water at room temperature. Mix until completely dispersed (about 3-4 minutes). Keep the suspension at rest (without mixing the product any more) for at least 15-20 minutes to reach the ideal consistency. The consistency of the preparation can also be adjusted by changing the amount of water used: a larger amount will provide a smoother consistency, a smaller amount will provide a firmer consistency. Consume preferably immediately after preparation, within 4 hours if stored at room temperature and within 24 hours if stored in the refrigerator. In case of excessive hardening of the product, which could occur following a prolonged storage time, apply a slight stirring to bring the suspension back to the desired degree of gelation.
Dosage and therapeutic indication
Two sachets a day or according to the doctor’s opinion are recommended. Take Aminoglutam ° Bel preferably between meals to facilitate its absorption.
Warnings and contraindications
Use under medical supervision.
The product is not suitable for use as the sole source of nutrition. Do not exceed the recommended doses for daily intake.
Not recommended for children under the age of 3.
Not for parenteral use.
Correct storage of aminoglutam sachets
Store at room temperature between 15 ° C and 30 ° C, away from localized heat sources, sunlight and contact with water.
Validity in unopened package: 36 months.
I find it interesting to know that Efthymios recovered using supplements rather than medication. I definitely think that AI has a big part to play in teasing out systems that are going wrong in these illnesses. Would be interested to know what tests would show up these deficiencies. Very difficult to find doctors willing to help in the U.K.
*Ammonia: check!
(Former) GP “probably a lab fault”
*Not tolerating alcohol since becoming Ill is probably 1 of the things we (pwME) ALL have in common?
*don’t know how this fits in but high cholesterol since my early 20’s (slender, not eating unhealthy, rarely alcohol) and not tolerating ANY drugs (statins or natural red yeast rice)
* beta amyloid 1-42 found in my csf recently.
Fits in w/ ER stress
Also: BBB disrupted.
Don’t know if this is a ‘long term’ effect if so many years of low grade inflammation (body & brain).
* EBV EBNA, VCA, IgG’s x 8 (!) of the highest normal range.
HHV6 in stomach biopsy (PCR)
CMV: very high levels of IgG’s
Tested numerous times & every time the come up abnormally high.
Reactivation? Don’t know.
But given Prusty’s viral protein findings in the brain this can’t be ok.
* still convinced neuroinflammation aka “microglia priming” & hypoxia are at the core of ME. Together with ‘body’ chronic low grade inflammation (liver, blood vessels, …).
* Been using AI myself lately (a lot) to try and connect dots. Have a lot of data (labs) over the years (22 yrs ME).
New research often ‘fits’ w/ these data.
To try & find answers & more importantly: “connections”, via questions & AI is taking a lot of energy & it’s moving too slow.
Also, data in AI are limited to 2021. So new research is left out.
Very very interested & curious as to what AI projects, like the ones discussed in this article, will be able to bring us in the future.
Also hoping more doctors will start using AI as a help to guide patients or to find out more about “infection driven chronic diseases”.
I think you need to be in a research study – or be a research study 🙂 Lot’s of checkmarks!
In the 1940s and 50s, high cholesterol was indicative of low thyroid function.
You can read Broda Barnes.
Have you tracked your temperature and heart rate?
This is a very promising approach, with one caveat (which applies to other research methods in ME/CFS, too). The research data generated so far mostly stems from comparisons between ME/CFS patients and healthy controls. Therefore, we do not know which part of the data is specific to ME/CFS (many findings could also apply to immune related / inflammatory disorders in general). For example, ist protein misfolding or ER stress or *you name it* a problem specific to ME/CFS? We do not know. And an AI-approach may not do away with this as it “learns” from possibly poorly specific data?
Right. ER stress is actually found in many diseases. I’m back and forth between whether its more helpful to find something found in many other diseases – and thus there may be treatments for it – and something that’s uniquely in ME/CFS – which reduces the likelihood that treatments are available.
Cort – I think, from your text, you meant to embed a network analysis image. But I don’t see one..?
Would look like this (from his Twiter): https://twitter.com/lifeanalytics/status/1678714877064380420
Nice writeups. I’ve been following Efthymios closely, this year, taking TUDCA and got probably a couple little bumps/correlations with addressing ER stress in my supplement regiment. Still investigating. 🙂
Oh jeez! Thanks for letting me know. I had them ready to go in and forgot – those are very illuminating images.
I also would like to know what he actually did to get free of this illness. This article is a teaser like the one about the man writing a book about how he got well and using this column to sell it to get details of what he did.
there where 2 times i thought given links to what he did, the previous blog Cort wrote on him. If that is what you mean?
There are no teasers (ach!) – the link to the recovery story was provided in the blog…
https://www.healthrising.org/blog/2023/10/02/efthymios-artificial-intelligence-chronic-fatigue-syndrome-recovery/
I found that one. Thanks, Cort. It was the nan who wrote a book about his recovery in the last article that gave no information on how he did it. Not blaming you. I read everything you post.
PRIS…Cort and others have provided link(s) that are posted above☝️ on his protocol
Excellent work again Court. I am amazed how well your brain seems to work. I would have to rest a lot in between writing complex matters.
I remember hearing that in Chinese medicine CFS is considered a liver and spleen issue.
See below:
“In most CFS patients, it is shown that the liver and spleen are affected. The liver in Chinese medicine regulates the emotions, stores blood and regulates the flow of Qi. When the liver is in disharmony, there is emotional depression, stagnation of energy and blood flow.
https://evergreencmc.com.au › chro…
Chronic Fatigue Syndrome – Evergreen Chinese Medical Centre”
Here is link that works on Chinesse medicine and CFS.
https://evergreencmc.com.au/chronic-fatigue-syndrome/
Efthymios, does the article in link provide any new insights? You may be very familiar with this already.
From the link above:
“From the perspective of Chinese medicine, CFS is considered a Qi deficiency. Qi, or life energy, gives a measure of the vitality of a person. The cause of CFS is considered to consist of various underlying and interacting factors. The typical TCM patterns for CFS include: spleen Qi and Yang deficiency; liver Qi stagnation and/or liver, spleen, stomach disharmony; heart blood and/or Yin deficiency; kidney Qi and Yang deficiency; phlegm obstruction and dampness retention; and heat toxicity.
In most CFS patients, it is shown that the liver and spleen are affected. The liver in Chinese medicine regulates the emotions, stores blood and regulates the flow of Qi. When the liver is in disharmony, there is emotional depression, stagnation of energy and blood flow. So, there is an actual depression of the whole system. The spleen transforms and transports the nutrients and fluids of the body and dominates the muscles. If the spleen is affected there is muscle weakness. The conveyance of body fluids is also impaired. Arthritic symptoms or swelling of the joints will occur. Many other organs are also affected as a result of the imbalance of the liver and spleen.
In Chinese medicine, all organs and functions of the body are connected in maintaining balance throughout the system. Therefore, when an organ is out of balance, it affects another.
Acupuncture works alongside Chinese medicine, acting on the body’s own subtle energy system to clear obstructions, and maximising the flow of energy and blood, in order to enhance the body’s own innate healing capacity, thus leading to an overall increase and vitality. Modern TCM treatment for CFS mainly focuses on adjusting immune dysfunction, regulating abnormal activity in the hypothalamic-pituitary-adrenal (HPA) axis, and detoxifying the system.”
Really interesting!
“The liver in Chinese medicine regulates the emotions, stores blood and regulates the flow of Qi. When the liver is in disharmony, there is emotional depression, stagnation of energy and blood flow. So, there is an actual depression of the whole system.”
Thanks!
What is refered to as the liver in traditional Chinese medicine is *not* what is refered to as the liver organ in western science/medicine.
And so with the other TCM organs..
I read that he has a patent on it. And I think by myself if patent (means owning) i a researchworld that has allready so much lack of money, so many sufferers for decades. If I would find something that helps, I would shout it from the roofs! Apology if I misunderstand a patent… I hope researchers will do it for “free” if still possible with the patent…
Sorry, to long, decades, to ill and to many people like that
Thank you for this comment. To make things clear, what is patented is the methodology that is being used to identify medical research targets. These identified targets are not under patent protection.
thank you for your answer! I just hope it is not to expensive (your brain 🙂 ), the methodology that is being used to identify medical research targets. So that reasearchers can easily pay for the patent in oh so underfunded desease(s).
May I ask you, have you plans to start a compagny with all you did to try to help us out of hell?
time is running out for so many of us… Or allready has…
ps I read your coment later then Cort’s comment on wich I answered.
I think it points to Efthymios’s confidence in this approach that he took a patent out in it in 2018 – before some of his findings showed up in later research. I hope he can put it to good use. 🙂
I really hope his patent does not cost to much for researchers….to use it or part of it. yes, if he starts a compagny for using it for us, I agree with the costs. That is normal. But I am maybe a “mistake” 🙂 , when i was still healthy and did not had found a job. I worked for free in an oldmans home and later with verry severe handicaped or ill people. That is my mindset… Until i found a payed job. I had to live from something. But this terrible desease, so many euthanasias, suicide, broken homes, violence, etc in the 25% group, etc I would give it for free. He is clever enough to find an other job. I hope you are right that he can put it to good use or does something on his own with it to help us out of hell. Time is running for so many of us…
This is great news. AI has incredible potential to solve ME/CFS and other “invisible medical conditions”
I definitely agree with liver issues as I’ve had symptoms of liver distress but as usual nothing shows up on blood tests etc.
I just read his recovery story and I may try some of the supplements but considering the protocol was designed specifically for him how do we know what’s right for the individual? I’ve tried so many supps over the years I should have bought shares in the company!!
“I’ve tried so many supps over the years I should have bought shares in the company!!” 🙂
While the first part of his protocol was designed specifically for him – the last part – which was created using an AI analysis of ME/CFS and other conditions – (and includes TDUCA) was designed for ME/CFS at large.
Thanks. I’m ordering them now. I have to keep trying until something works or helps even a little.
do you mean this supplements?
TUDCA – I started with 250 mg and gradually upped the dose to 750 mg, providing 250 mg every 8 hours in order to ameliorate endoplasmic reticulum stress (and subsequent unfolded protein response events) and also support liver function. TUDCA, a supplement and bile acid derivative, is another “version” of a medication called Ursodeoxycholic acid aka ursodiol: the liver produces bile acids which are metabolized in the intestines into TUDCA. These bile acids digest fat-soluble vitamins, break down food and cholesterol, help fight off pathogens, and regulate our metabolism. In an energy-intensive process, most of the bile in our body ends up getting reused. (More on bile acids and the liver in the next blog on Efthymios’s work on artificial intelligence and ME/CFS.)
Methylcobalamin (B-12) (1000 mcg) – to support methylation)
Methylfolate (200 mcg) – to support methylation)
Taurine (500 mg) – to help in conjugation of bile acids via CYP7A1, regulate NMDA / glutamate excitotoxicity, and also upregulate PGC1-α (for mitochondrial biogenesis)
Vitamin C (250 mg) and N-acetyl cysteine (100 mg – I couldn’t tolerate more) – antioxidants to minimize oxidative stress
Ubiquinol (100 mg) – provides intermediate metabolites for energy production
Zinc (25mg) – which has been low whenever I tested it
Selenium (50 mcg) – (combat oxidative stress)
Vitamin K2 (As MenaQ -100 mcg) – (combat oxidative stress)
Choline (500 mg) and important cofactors such as FMN (Flavin Mononucleotide) (10 mg) (combat oxidative stress)
D-Limonene (250 mg) twice a week to induce P450 (liver support and detox) (Caution: this may affect drug metabolism)
like from ubiquinol i can totally not sleep… sadly enough because it helps a bit… some others no problem, many i do not know. But maybe like with the ubuiquinol, we are to heterogenous. Even non ME/cfs people if i looked up the revieuws of co q10 on webmed.com , there where also people that could not sleep from it.
Liver Question:
How do people with ME/CFS do after liver transplants? Any studies or anecdotal reports?
I seem to be an outlier in this group. Most of what I’ve read here doesn’t seem to apply to me! ( of course, maybe it does, and I just dont know it). In any case, I can’t just go off on a wild goose chase and start trying random things!! I can describe my symptoms pretty well, and my present hypothesis is that post viral syndrome makes the most sense! (post polio syndrome). I do think that malfunctioning mitochondria has a lot to do with my severe illness! Every thing described involves complex biochemical processes. I don’t know if each of us is unique or if there ate groups of us which follow similar patterns!! I’m 84 yrs old and just want some peace and freedom from pain! Many thanks to all of you who devoted so much effort to helping diagnose and treat our ailment(s)
This is extremely interesting to me in terms of the correlation between liver injury (caused by viral infections, etc.) and illnesses like ME/CFS. The doctor who diagnosed me at age 40 suspected I had had ME since hepatitis at the age of 7. This would fit his hypothesis. I will look forward to more information in the future – though as I am 72 now, it may come too late for my quality of life.
Never too late Diane. Every day is another day..
I also am post polio having had polio as a child and i considered that my symptoms might be post polio or cfs; hard to know..from what i understand post polio occurs about 40 years old and i am past that.. Maybe aI will give us some answers; For me, I always keep trying..good luck (seems so feeble to say that..)
And good luck to you too Dorothy. There are so many things to try, and they all cost more money! I hope some insights come in time for both of us.
In the meantime, I have recently started on Natural Dessicated Thyroid and that has restored some of the energy I lost in December 2019. It’s good to be smiling and laughing again, and even a bit of singing around the house!
Very interesting read Cort Johnson.
Thanks for your work on this.
I did read a book and follow the guidance when I had ME/CFS & fibromyalgia: Liver Cleansing Diet: Love Your Liver and Live Longer by Dr Sandra Cabot
I see there are lots of books on this topic on Amazon.
As a long time (20+years) CFS “sufferer”, I find that I experience many of the symptoms you talk about. However, i find my symptoms getting worse. I appreciate all the findings and try to follow everything . Thank you for all your work.
Dorothy, Thank you. I must stress again that the regimen I described was tailored according to my symptoms. Whatever worked for me does not mean in any way that it will work for someone else. However, we may be finally finding certain concepts such as inflammation, oxidative stress, mitochondiral dysfunction, liver dysfunction,endoplasmic reticulum stress, autophagy, calcium metabolism (list non-inclusive) that may be leading to a vicious cycle we are seeing in these syndromes.
Thanks for this article, Cort, that is indeed exciting and promising news. We are many who are curious to learn more about Efthymios’ treatment protocol, if you could elaborate a bit more on that?
I’ve had ME/CFS since 2009. Last year I was, to my big surprice, diagnosed with non-alcoholic fatty liver disease, and I am not overweight. A doc said it could be due to some og the meds I take (particularly citalopram), but now I know it could also be due to ME itself.
Well you certainly seem to fit this scenario Anna! There is a link to Efthymios’s recovery story in the blog. You can also find it here – https://www.healthrising.org/blog/2023/10/02/efthymios-artificial-intelligence-chronic-fatigue-syndrome-recovery/ Good luck!
I fit in here as another with long term ME/CFS, 27 years since I “fell down” with it mid-40’s and had to give up my active work and limit so much else. Now 74 and “the water is draining out of the bath”, but want to say I am thrilled that Efthymios found a liver connection.
Liver: At 31 I had a severe infection to my organs, afterwards just labelled “peri-hepatitis” after which I could not tolerate alcohol. Had more chronic pain in muscles than most people do too. I relieved this pain only with stretching and exercise then started using ibuprofen too. Half maximum dose, but still, sometimes daily. Later I switched to tylenol, also for years. While never taking its maximum dose, perhaps my liver was harmed by this frequency. For me I was so grateful to have a way out of pain all day. Not acute, but constant, constant aching. Next thing that occurred was intolerance to both of them. Even one halved pill of Ibuprofen with food feels like it is burning a hole in my stomach. And even a half dose of tylenol first puts me to sleep for a few hours then I wake up feeling hungover. This is an acute feeling for half an hour so I do not use tylenol either. A neurologist prescribed Gabapentin which thankfully relieved the excess aching He added that it does not burden the liver either with the detox job, but that “the kidneys kick it out”. So much for my liver, except that chocolate does not agree with me either, if that is connected.
Misfolded proteins: Last year was diagnosed with an alpha-synucleinopathy. As it doesn’t look like Parkinson’s yet, emphasis on yet, this was called Pure Autonomic Failure. I certainly have had a serious, disabling degree of dysautonomia for years.
So maybe my physical history here illustrates the course indicated by Efthymios’s research, starting with liver damage of some kind.
We need better liver tests, assessments and analysis to track this down better and treat it. Also a much more conservative approach to the supposedly safe pain killers, and any other drugs, which impose on the liver too.
I would also rule in/out APS also known as Hughes Syndrome after a Doctor in the UK a blood clotting auto immune blood test.
Men get this as well & one can be also Negative on Lupus testing
I am soooo excited to see people talking about the liver in ME/CFS! My daughter only recently received an official diagnosis of ME/CFS but I believe it all started with liver injury from Epstein Barr over seven years ago. I also feel like everything that visibly improves or worsens her condition is all liver related as well.
When my daughter was twelve she was training in competitive dance and all the girls caught this awful virus- one girl didn’t come back to practice for a couple months. My daughter didn’t get that sick initially, but she soon started experiencing debilitating abdominal pain. Ultrasound and lab work revealed that her liver was enlarged and her ceruloplasmin levels were low. After clearing major things like Wilson’s Disease, we were told that her lab results were insignificant, even though her health continued to deteriorate. Her former life energy seemed to just drain from her, her colour changed, her abdominal pain became chronic, she had to spend more and more days in bed, and she developed chronic infections, POTS and migraines.
Her condition continued to worsen after suffering a concussion and neck injury from a car accident, followed by the sudden development of scoliosis about six months later. We have more recently found out that she has a mutation in her ELN gene (as well as her MTHFR gene) causing weak connective tissue, which would explain such a sudden onset of scoliosis. Considering the predisposition to developing ME/CFS within the hypermobile community, I find it interesting to note that in Chinese Medicine, treatment of the tendons(connective tissue) focuses on treatment of the liver.
A year after that, my daughter had a terrible response to a Hep A/Hep B vaccination (liver-related vaccinations), which left her in bed for weeks and severely affected her cognitive function. She felt like she couldn’t “think” anymore. Over the next year she deteriorated further to the point that she became completely bedbound, unable to read, write or draw, and at her worst, even have us in the same room.
We still didn’t know about ME/CFS at that time, and only stumbled upon it after a strange trip to the ER for tachycardia, which led to my daughter receiving an adenosine IV to stop and reset her heart. The strange part of the experience was that the adenosine actually made her feel normal again for about 24hrs. No pain, no heaviness, normal energy, she could think clearly again- we couldn’t believe it. It was only in researching adenosine that I started reading about the dysfunctional ATP production that exists in ME/CFS and we started to piece things together and pursue an ME/CFS diagnosis.
The other interesting thing I found out about adenosine is that it seems like there was a time when it was used to treat porphyria; a liver disease in which biosynthesis of heme is impaired resulting in excessive porphyrins and precursor buildup. (They currently use heme treatments to reduce porphyrins during porphyric attacks, and I’ve seen some research in India on CFS symptoms being induced in mice and alleviated by heme treatments). My daughter seems to have consistently raised coproporphyrin numbers on labwork, which although not indicative of porphyria on its own, can be indicative of other low grade liver disease.
Also, as I went down the rabbit hole of supplement research, we started trialling Zinc and B6 as a Pyroluria protocol for her (which is essentially a less severe form of the heme biosynthesis dysfunction that exists in porphyria, only recognized in natural medicine and treated with these supplements to replenish the deficiencies assumed to develop due to excessive production and excretion of porphyrins). She started to feel less heavy and stop crashing. Zinc was a game changer for her. Almost all of her digestive issues resolved and her chronic abdominal pain disappeared completely. The day after we introduced B6 she was able to come downstairs for the first time in months and carry on a conversation at length. Early this year she started to read again, listen to music and be able to watch movies (but interestingly only in her second language at first because her first language was still too stimulating to her brain).
More recently, as I learned of the Tulane University study we started supplementing with methylated folate and B12, and with very careful pacing, my daughter was able to go on a short trip to Asia to see family without crashing.
Since receiving an official diagnosis of ME/CFS and starting low dose naltrexone for neuroinflammation, she has seen further improvements especially in her cognitive function. She’s able to do more in English now(first language) and she is now studying multiple other languages (mostly from bed) and can come downstairs to spend time with us and go out for short outings. We had an ultrasound performed a few weeks ago and after seven years of enlarged liver it has suddenly decreased to a normal size again. We don’t know why, but we wonder if it was a positive effect from the anti inflammatory nature of the naltrexone.
My daughter is nowhere near being recovered from ME/CFS; she still spends a lot of time in bed resting, and her circadian rhythm is all over the place, but I believe that the liver is very connected to ME/CFS. We have observed similar patterns in diet to what Efthymios mentioned in his recovery story, and my daughter always feels better after blood draws(which reduces iron in the body- iron can’t be used efficiently without proper liver function and sufficient ceruloplasmin levels). We look forward to trying some of the other things Efthymios found helpful like TUDCA, and I’m curious if he’s looked into anything porphyrin related in his research of the liver?
Thank you so much for sharing Efthymios, and thank you Cort for always keeping us in the know and connected!
So interesting! Thanks for sharing your daughters story Holly. She’s lucky to have such committed parents! Here’s to figuring out more pieces of the puzzle and your daughter’s continued recovery 🙂
Thank you Cort! You’re helping all of us piece things together xo
There is a type of porphyria that is ‘acquired’ and not ‘genetic’.
The treatment is a high carb diet.
Back when the incidence of pellagra was high in the States, some doctora measured high porphyrins in theburine of pellagrins during their pellagra attacks. They would normalize when they didn’t have pellagra anymore.
Pellagra has many new names in contemporary medicine. That acquired porphyria might be one of them.
Have you tried a little niacinamide and see how your daughter responds?
You might like reading Ray Peat and help your daughter further heal. I think when you are young, like her, it is easier to do through foid and nutrition.
Goodluck!
Pffftt… spelling mistakes… that was food and nutrition in the last line.
And the ‘high’ carb diet is for all the porphyrias, the genetic ones as well.
Thank you! We do notice that my daughter does best by carb loading whenever she is most symptomatic. I will check out Ray Peat!
Perhaps keeping her carb intake high and experimenting with the protein load and type, will raise her metabolic rate and prevent her flares to begin with.
The umbrella term carb is misleading as if they were all the same, just as if fats were all the same or all proteins were the same.
Fruits are a good source of energy sugar minerals
The rehydration salts people often write about taking/helping for POTS, ME/CFS, etc – the WHO mix that’s been written about here before – that’s marketing.
Fuit juices are natural sources of those minerals plus the fructose. You can salt them a little, just a pinch without modifying taste.
Your original, natural Gatorade without refined industrial byproducts
Godspeed
Holly, Thank you very much for sharing. All of the conditions you mentioned (Porphyria, Wilson’s Disease) have been on my radar (I am not sure about Pyroluria although I came across it while researching). I would say that *any* condition that can impair liver function should be ruled out and unfortunately there are many of them. I am so happy to read your daughter is feeling better and I hope this continues.
Thank you:)
All of this resonates with Ben Lynch: https://www.youtube.com/watch?v=CK101rZ3mEE
For ammonia to be conjugated into urea and thus excreted via urine, ATP is needed.
In a low metabolic state – i.e. hypothyroidism – ATP is low, ammonia accumulates.
Ammonia can also be due to tissue catabolism. The oxidation of glucose produces more ATP than of fat or breakdown of protein. If you don’t eat enough sugar, your body will turn to its own tissues to produce it. *Anyone* in the sports world knows this. Doctors don’t.
I used to sweat out ammonia for over a decade. My blood urea levels were also low. Upping my sugar intake helped a little. Thyroid supplementation is what vanquishes it completely. If I start smelling ammonia, I know it is time to adjust my thyroid dose.
There are a number of urea cycle disorders, and problems converting ammonia to urea, it builds up dangerously high. Fatal for many. Others live thanks to liver transplants. Part of the treatment is to avoid proteins and high carbs. In fact, many of the inborn metabolic disorders have the same dietary recommendation. same for the porphyrias. Often it is when a person does a high protein / low carb diet that the disease makes an appereance. I wonder how many of the genetic disease would not show up or as severely if proper metabolism was addressed (via diet, because sugar, and protein, and thyroid)
I happen to have a genetic defect for an urea cycle disorder. I spent some time in this community; oddly every disease has similar symptoms, akin to fibro/me/CFS. I really don’t understand the difference much, except that maybe one has more joints affected, another more mind issues, etc. Also, I asked if they could smell the ammonia. And many didn’t, even when they were unresponsive and had to be hospitalized and levels sky high in blood tests.
So I don’t know what that is – that those have an ‘official’ diagnosis of an urea cycle disorder maybe can’t get rid of their ammonia, while even though I could have had the same problem, my body was able to expel some of the excess through sweat. So maybe the urea cycle disorder is not only about an urea cycle defect, add to it the inability to excrete whatever builds up. I don’t know.
Which also – you could have high levels of ammonia and not know, because it doesn’t come out in ways you can recognize it.
[Spelling mistakes. Fat fingers on phone keyboard…]
Would love to hear his story of how he got rid of his ME/CFS.
This is the first time I have heard anything about the liver in relation to ME CFS. That’s very interesting to me because I feel like all my problems started around the time I had to get my gallbladder removed because of gallstones and as we know, the gallbladder is connected to the liver… so I wonder if it could be causing issues for me. Now I would love to see research data on how many people with ME CFS have also had their gallbladder removed.
Thank you for bringing this up. The following slide is from a presentation I made in 2018 to several ME/CFS researchers. If you look at the results from machine learning algorithms, “cholecystectomy” can be seen among these results : https://twitter.com/lifeanalytics/status/1699133771235070406/photo/1
Oh wow! Thank you for your reply, Efthymios. That’s so cool that you found something that might connect ME CFS for liver issues. I wish I could understand your slides better to know what the results were exactly for people who had a cholecystectomy. I’m not sure if the slides just didn’t show all the data, if it was just too technical for me to understand, or if my brain fog from ME CFS is making things just too difficult for me, but I didn’t really know what I was looking at other than I think it was basically just showing that there seems to be a correlation between having ME CFS and having your gallbladder removed. Also, I read the article about your recovery and I am very happy for you finding a solution that gets you out of this misery. I have many of the same issues you had including having the MTHFR gene, but also you had many I didn’t have. Did you say you had your gallbladder removed? I’m not sure if I saw that.
Terra, no I did not have my gallbladder removed. The results suggest that the AI system has identified another potential factor (cholecystectomy) that needs to be evaluated by medical community. For example, we need to ask whether ME/CFS patients have an increased percentage of cholecystectomies when compared with the general population. Also, can the formation of gallbladder stones (and subsequent gallbladder removal if necessary) tell us anything about ME/CFS itself?
That would be such a great area to research! Btw, I’m going to ask my doctor next week during my physical exam what she thinks about me trying out some of those supplements you take for liver detoxification. I’m especially interested in the TUDCA one. Thank you again for sharing your story and research with all of us.
I would also be interested in this. My issues also started around gall bladder removal.
Efthymio –
Have you tried running your search engine on endotoxin/LPS and liver?
I had pain in my liver area for about 10 years. My liver enzymes kept dropping year after year too, they started getting dangerousky low but which doctor understand this is a lroblem? The algorithm trains them to look for high levels = danger only.
I did not address my liver specifically to heal. In addressing all the nutritional and hormonal deficiencies I had, my liver improved.
Kay T, no I did not have the concepts you mentioned. I will add them, Thank you
I forgot to say to search for this outside the ME/CFS literature.
I think there has been only one or two ME/CFS people that mention LPS. Cort had a blog within the last months.
Alao, my cholesterol always tested low – the sicker I became, the lower it went. And again, doctors are not trained to flag this or be concerned, only high levels. I even had doctors ‘congratulate’ me on my low cholesterol. I shudder when I think about this.
There is a correlation between low cholesterol and negative prognosis with any disease.
Usually it is indicative of high LPS load or low carb consumption. This affects liver.
Hypothyroidism also affects liver function. You could also look through the literature for that. Cort has a few blogs, Dominic Stanculescu wrote them and also published papers.
If you are interested in know how ALL of this connects, you can read Ray Peat. He has written about many of the ME/CFS findings before they were found – because they already figured in scientific literature.
Help me understand better the Xanthine oxidase issue. I’ve heard many pwme get better when using milk thistle. Milk thistle seems to inhibit xanthine oxidase, would this make sense coupled with what Efthymios found?