


Janet Dafoe interviewed Robert Phair for the Open Medicine Foundation.
Janet Dafoe has been doing an informative series of videos on the Open Medicine Foundation’s work. Her patient-centered approach – she often interrupts a researcher engaging in “research speak” to clarify in simple English what’s going on – is refreshing.
At the end of 2022 and the beginning of 2023, her two-part series with Robert Phair – the creator of the IDO Metabolic Trap hypothesis for ME/CFS – focused on his latest approach – the Itaconate Trap Hypothesis.
The Itaconate Shunt hypothesis – with its potential ability to explain so much in ME/CFS (energy production problems, strange metabolomic results, post-exertional malaise, brain fog, immune issues) – provided a compelling idea.
After the Open Medicine Foundation provided vital support to the development of the hypothesis, Vinod and Neeru Khosla’s Amar Foundation in San Jose, CA jumped in to provide funding to test it in Chris Armstrong’s lab at the Open Medicine Foundation’s Melbourne Collaboration at the University of Melbourne in Australia.
Vinod Khosla, a co-founder of Sun Microsystems, and his wife, Neeru, created the Amar Foundation in 1987. In 2011, they were one of the early adopters to sign onto the Giving Pledge created by Warren Buffet, and Melinda and Bill Gates, which commits wealthy individuals to give away the majority of their wealth during their lifetimes or in their wills.
The Mind Meld
Robert Phair PhD is the cofounder of Integrative Bioinformatics Inc., a computational biology consulting firm that has been taking a systematic approach to the modeling of biological systems for over 20 years. Phair got interested in ME/CFS when he met a neighbor with the disease at Stanford and began working with Ron Davis to find solutions for ME/CFS in 2016.
In late 2019, during discussions with Chris Armstrong – the Open Medicine Foundation’s metabolomics expert – and since 2020, the leader of the Open Medicine Foundation’s Melbourne Collaboration, Phair and Armstrong puzzled over the “ammonia problem” in ME/CFS. Armstrong and others found that ME/CFS patients’ cells were preferentially using amino acids instead of the body’s preferred sources – glucose or fatty acids.
Amino acids are not a preferred energy substrate for a couple of reasons. Among them is that they have this pesky nitrogen atom attached to them that needs to be taken care of. The body usually eliminates the nitrogen using a variety of “safe” forms, but ME/CFS studies have not found increased levels of these safe forms. That suggested “unsafe” forms of nitrogen such as ammonia or peroxynitrite were building up. These two highly reactive compounds can, among other things, wreak havoc in the energy production systems that power our cells.
During the pandemic, when Phair had shifted his work to understanding the innate immune system, he uncovered an innate immune enzyme called CAD which he felt might help explain the ammonia mystery. With support from the Open Medicine Foundation and the Amar Foundation, Phair got to work. Using a modeling tool created by Integrative Bioinformatics Inc., Phair had a potential answer by Sept. 2021 to the amino acid/ammonia problem in ME/CFS – and the Itaconate Trap hypothesis was borne.
The hypothesis is particularly compelling because it potentially ties together an infectious insult, a hit to the energy production system, brain fog, post-exertional malaise, and immune dysfunction.
The Itaconate Shunt (or Trap) Hypothesis Pt. I
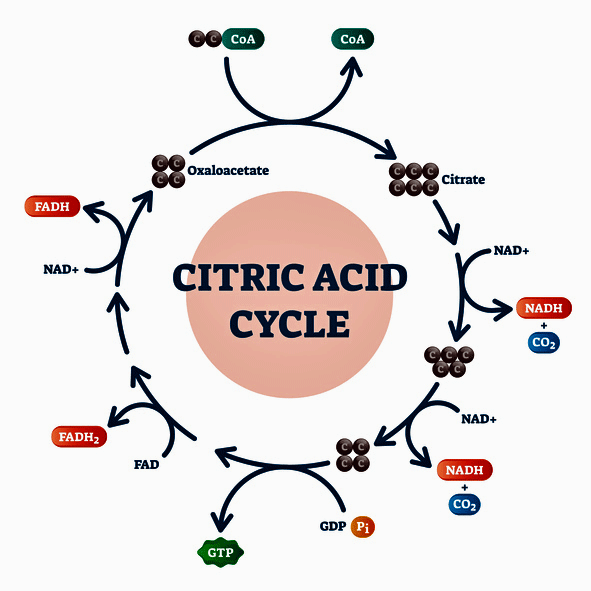
The important thing to note in this diagram are the areas just outside the circle where NADH and FADH2 are produced. They provide the electrons that power the electron transport chain where ATP is produced. The itaconate shunt blocks them from being produced.
The first thing to know is that a variety of transformations occur within the citric acid, or Krebs, cycle in the mitochondria which ultimately produce substances like NADH and FADH2 which provide the electrons that power the electron transport chain to produce ATP.
It all begins outside the mitochondria, though, with energy substrates the Krebs, or TCA, cycle uses to produce NADH/FADH2. The cycle’s preferred energy sources are glucose and fatty acids. Notice the central role that acetyl-CoA plays in the cycle.
- Glycolysis converts glucose to pyruvate, which gets converted to acetyl-CoA
- Fatty acids – get into Krebs cycle via conversion to acetyl-CoA
The Krebs cycle can use amino acids as well. Amino acids, though, get into the Krebs cycle in an entirely different way and at a different point.
An Infection – Energy Disruption Link
So far, so good, but then comes the itaconate shunt – which is initiated by our old “friend”, the innate immune system. The innate immune system is an ancient immune response found in all vertebrates that quickly scrambles to hold an infection in check long enough for the adaptive immune response to mount a hopefully devastating pathogen-specific attack a couple of days later. The innate immune system can also be activated by stress, injuries, or environmental toxins.
One of the many factors that the innate immune system produces is called cis-aconitate decarboxylase, or CAD. In just the second step of the Krebs cycle, CAD interrupts it – sending plunging it down a different path – one which effectively turns off its ability to produce abundant amounts of energy.
First CAD alters cis-aconitate to itaconate, which then gets catalyzed by an enzyme in the Krebs cycle called STK (succinate thiokinase) into something called Itaconyl CoA.
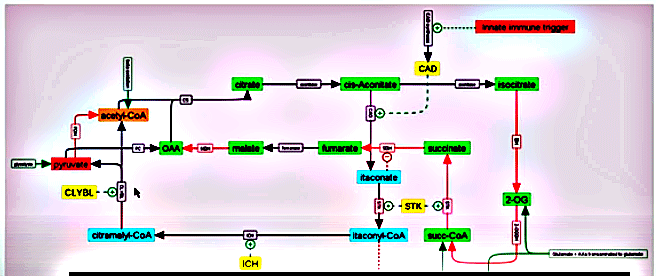
The itaconate shunt – in blue – bypasses the energy-producing portions of the Krebs cycle.
Remember acetyl-CoA? The Krebs cycle normally breaks it up – allowing the cycle to move forward. In the itaconate shunt, though, STK loads CoA onto itaconate thus turning the Krebs cycle into an energy sink instead of an energy producer. Instead of the Krebs cycle producing ATP, ATP is actually lost. The itaconate shunt, then, effectively bypasses the energy-producing steps of the Krebs cycle.
This does not necessarily mean that energy production is completely stopped – some cis-aconitase is probably still getting through – but it’s probably severely inhibited.
Our cells have produced a backup energy system, however, called the GABA shunt.
THE GIST
- Janet Dafoe interviewed Robert Phair twice on his Itaconate Shunt hypothesis for the Open Medicine Foundation at the end of last year and in the beginning of this year. This blog is from the first interview.
- The Itaconate Shunt hypothesis is compelling because it potentially ties together an infectious insult, a hit to the energy production system, brain fog, post-exertional malaise, and immune dysfunction. First funded by the Open Medicine Foundation, work testing the hypothesis is now being funded by the Amar Foundation created by Vinod and Neeru Khosla.
- The roots of the hypothesis began during discussions between Robert Phair and Chris Armstrong, the leader of the Open Medicine Foundation’s Melbourne Collaboration in Australia. Armstrong and others had found that people with ME/CFS were preferentially using amino acids instead of better fuels like glucose and fatty acids to power their cells.
- The increased utilization of amino acids should have produced high levels of nitrogenous byproducts in their blood. The fact that they weren’t present suggested that the safe breakdown of amino acids wasn’t happening and that toxic byproducts like ammonia were being produced instead.
- Phair glommed onto a possible reason for this during the coronavirus pandemic. He found that an immune enzyme called CAD that is produced during an infection can produce something called an “itaconate shunt” which causes the energy-producing cycle in the mitochondria to short-circuit.
- In fact, it’s worse than that. Not only does the energy-producing cycle (the TCA/Krebs/citric acid cycle) get whacked but the itaconate shunt turns it into an energy sink. Instead of producing energy, it actually draws energy from the cell.
- It seems bizarre to turn off or inhibit energy production during an infection but it has a purpose. Because the pathogens that infect cells use the cell’s energy to produce more pathogens it’s thought that the itaconate shunt temporarily shuts down the cell to restrict pathogen replication long enough for the next phase of the immune system – the adaptive immune system – to gear up to wipe out the pathogen.
- Phair proposes that instead of lasting for a few days, ih ME/CFS the itaconate shunt becomes permanently turned on.
- Our cells have produced a backup energy system, however, called the GABA shunt – which could explain the preferential use of amino acids by ME/CFS patient’s cells. Unlike the other parts of the Krebs/Citric acid/TCA cycle the GABA shunt uses amino acids for energy and is not affected by the itaconate shunt.
- The GABA shunt, though, produces only about 40% of the normal energy produced by our cells – and it comes with a problem – it leaves behind nitrogen byproducts that need to be broken down. As noted earlier, studies suggest that our amino acids are not being broken down safely – possibly resulting in high levels of ammonia – a toxic byproduct that can, among others, affect energy production.
- The hypothesis is being tested by Chris Armstrong at the Open Medicine Foundation’s Melbourne Center and by at least one other group of researchers.
- In Pt II Health Rising will cover why the itaconate shunt may be becoming chronic in ME/CFS and where we are now with the hypothesis.
A Defense Strategy
The great question that arises is: why? Why would we have hardwired into our cells a way for the innate immune system to turn off energy production during an infection? The shunt must serve a purpose, and it does. It’s a defense strategy. As the itaconate shunt whacks the energy production of our cells, it’s also doing a number on the pathogens that have infected our cells. They need energy, after all, to reproduce – energy they get from our cells. With the cell’s energy centers shut down, the pathogens have trouble replicating – giving our adaptive immune system a chance to rev up and attack them with pathogen-specific immune hunters.
While Phair doesn’t mention it in his talk, this energy shutdown during an infection seems reminiscent of Robert Naviaux’s Dauer hypothesis. It also seems to jive with the idea of “sickness behavior” in which symptoms like fatigue, brain fog, and other flu-like symptoms immobilize an individual and keep her/him from spreading an infection.
The idea is that instead of staying on for a couple of hours, or at most a couple of days, the shunt gets turned on permanently in ME/CFS.
The Workaround – the GABA Shunt
But what about the preferential use of amino acids to produce energy we’re seeing in ME/CFS? We’ve had no answer to that. It turns out, though, that the Itaconate Shunt hypothesis provides a convenient answer for that – although it’s not a pretty one.
As we’ve seen, both glucose and fatty acid metabolism in the Krebs cycle depend on CoA – the substance that the itaconate shunt essentially takes out of the picture.
Of course, our cells have a workaround – a kind of failsafe to ensure that at least some energy is still being produced. An alternative to the normal workings of the Krebs cycle – called the GABA shunt – is available.
The GABA shunt uses four enzymes already present in the Krebs cycle to complete the cycle in the absence of CoA and ultimately produce ATP. It’s only about 40% as efficient as the CoA-powered Krebs cycle, but you do eventually get some ATP out of it.
With the GABA shunt, we have a possible answer to the strange pattern of the preferential amino acid utilization issue seen in ME/CFS. If the Itaconate Shunt hypothesis is correct, our cells are mostly turning to the only available substrate left – an intermediate amino acid called glutamate – to power their engines.
The hits don’t stop with energy production, however. Using high levels of glutamate to feed our mitochondria has its consequences – among them leaving the brain high and dry regarding its essential energy substrate – glutamate – potentially producing brain fog, problems with memory and learning, etc.
Ammonia

Chris Armstrong is checking whether higher than normal levels of ammonia are being produced in ME/CFS.
With the GABA shunt, we get to what started this whole thing off – ammonia! Both the Itaconate Trap hypothesis and ME/CFS metabolomic studies suggest that our cells are probably loaded with glutamate. If the hypothesis is correct, amino acids are the only really usable energy substrate we have left.
That, however, leaves us in a tricky situation. When we exert ourselves and use up our ATP, something called ADP is left over. (Instead of adenosine triphosphate (ATP) we’re left with adenosine diphosphate (ADP).) ADP is the normal byproduct of energy production.
ADP, though, triggers the breakdown of glutamate, which results (in part) in the production of ammonia – a highly toxic substance. This is not usually a problem because: a) the Krebs normally runs mostly on glucose and fatty acids and doesn’t produce much ammonia, and b) healthy people can safely break it down anyway. Because ME/CFS cells may be top-loaded with ammonia, and perhaps because people with ME/CFS have trouble breaking it down, Phair and Armstrong believe high levels of ammonia may be present.
This is “one of the many ways” Phair believes that ME/CFS may get going. Chris Armstrong is designing carbon 13 tracers to see if the itaconate shunt has become chronic in ME/CFS.
Note that the itaconate shunt is not a hypothesis – we know it happens in the early stages of an infection. The Itaconate Shunt or Trap hypothesis, though, proposes that it has become chronic in ME/CFS. The next part of Janet Dafoe’s interview with Robert Phair, in Pt. 2, tackles why he believes the shunt may have become chronic in ME/CFS.

We love communicating the creative ways researchers are attempting to explain these illnesses.
Health Rising’s BIG (little) End of the Year Drive
Thanks to the over 325 people who have supported Health Rising thus far!
Something is going to explain ME/CFS and post-infectious diseases like it at some point. Whether it will be the Itaconate Shunt hypothesis or something else no one knows but highlighting the creative ways researchers are coming up with to explain these diseases is something we love to do.
If learning about the latest efforts of researchers to understand our diseases inspires you as well please support us in a way that works for you.






Hi Cort,
This is the most logical presentation I have seen yet about a possible CAUSE of Chronic Fatigue. An interruption of the normal Krebs cycle in the mitochondria caused by infection. Makes sense in MY case which has lasted 24 years, and has gotten worse with major infections, including last August’s COVID.
But as usual, my question is: is anyone working on a DRUG or REMEDY for this condition?
At age 81, my life is hardly worth living without some kind of improvement.
Phair notes that his main goal is finding treatments for ME/CFS. As to what they might be I don’t know. Getting to a core finding would be a big help.
Lawrence, To quote Ron Davis, “It’s possible that the only way to test a cell-autonomous theory of ME/CFS pathophysiology (such as the itaconate shunt) is to choose a drug based on the theory and trial it in ME/CFS patients.” We are following this line of investigation in parallel with our efforts to establish the molecular basis of ME/CFS pathophysiology. There are several candidates. We are with you.
Thanks for your hard work and persistence robert
Thank you Robert.
It’s great to hear you are thinking of potential drugs, as one of the most important things for us patients stuck and suffering in this horrible life long disease trap, is hearing that existing drugs can be researched. Because new drug studies take many years to ever get to the patient. And that’s a wait that is too much to handle.
Knowing there’s existing drugs out there with potential, keeps us hanging on to hope.
There’s an NGO called ‘EveryCure’ that look for FDA approved drugs for off label repurposing in other diseases. Perhaps contact them as they are good at sourcing, and know how to get through the legal hoops with the supply of these medications for researchers
https://everycure.org/
I noticed immediate and sustained improvement with 10mg Otezla. Along with LDN, metformin, and NAC. I hope it sustains and that perhaps they’ll work synergistically and cumulatively.
Dr Phair thank you so much for working hard to understand and treat ME/CFS. I’m currently severe and am bedbound 90-95% and knowing people are actively working to combat this horrific disease keeps me hopeful. I’m based in the UK and would be open to trialling drugs here via one of the practitioners I see if that’s ever of use/interest? (I saw you mentioned in another comment that certain drugs are licensed in the EU that aren’t in the States.)
Lastly, I wondered whether the Itaconate shunt hypothesis and Dr Naviaux’s purinergic theory complement one another? I don’t have enough understanding of the science to work it out but have been wondering!
Again, thank you!!
Merry Christmas Robert phair, what a wonderful comment to read on new years eve 🙂 You give us so much hope. I love how dedicated your group is, and how focussed on the whole disease as a process, trying to further research that joins the dots. Thank you for everything you guys are doing. Good luck with your research this coming year. Sending you kind thoughts.
Hi Robert Phair. Here is fascinating condition that is sometimes concomitant with CFS as in my case, whose study I am certain could shed light into shunt initiation/worsening.
It is called POIS. Post Orgasmic Illness Syndrome. A percent of men with CFS also have this condition. And many people with POIS do not have CFS. But I believe they go through the shunt loop because of the symptoms. After the loss of seminal fluid (regardless of how) we experience a crash that it is reminiscent of an intense CFS crash the lasts 2 to 3 days on average. Note the time period coincidence with the expected duration of the shunt under more normal conditions. The POIS “crash” ALWAYS happens. Things that help are the same ones that help CFS.
I take immunocal to compensate. It is an undernatured whey protein that contains Glutamine and Glutamate. Please note that Glutamine is found in abundance in semen.
After 30+ years with both CFS and POIS I am convinced that POIS is a short version of CFS. It could be that people with POIS repeatedly trigger the shunt, and since the body prioritizes reproduction, it diverts Glutamate/Glutamine to replenish semen and thus takes it away from an already deprived body. Just thoughts here.
The predictability of POIS can make it a good process to study. Since people with POIS schedule sexual activity. And typically cycle every 1, 2 or 3 weeks, as they choose. So people are scheduling “crashes”. So the body systems could be monitored, before and after the event.
A POIS crash, begins between 1/2 hour to a few hours after the event. The symptoms are severe fatigue and weakness, severe concentration and memory problems, flu like symptoms, reduce social skills. Word search and reduced eloquence. Severe PEM, increased POTS, irritability, anxiety, etc. Not a full list.
This is a recognized medical conditions under NORD, and it has an orphan status. So the many thousands of people who have it are eager to contribute to research by volunteering. Here is a forum where you can learn more about the condition.
https://poiscenter.com/forums/index.php
My username is MrRaba. If you provide an email I can put in contact with members.
Hello Robert, just added to the end of this comment section a link to a Chinese-American CFS treatment study measuring lactic acid and blood ammonia levels. Also sent it your way via OMF email adress, but as the automatic reply said they might not reply due to high volume of emails, I am sending this message, too. Kind regards!
I agree, it’s definitely the most beautifully simple and logical cause hypothesis.
I quite like it, enough to have been bringing it to all my providers over the last couple weeks, and you can literally see the lightbulb go off in their brain every time! I think that’s probably a good sign about finding a treatment, medical providers are getting excited about it and it makes sense to them.
Anecdotally, I am definitely noticing patterns that line up, there is definitely more ammonia in my urine when i overexert and i have had success with consuming protein when my blood pressure is decent and avoiding it when my POTS is flaring up (logic being that since low blood pressure slows down the removal of ammonia, not to give it any “fuel” during tbose times, but other times give my body the stuff it’s actually using for energy). So it may not provide a direct treatment but i have definitely experienced success making decisions informed by it.
I’m considering raising my gabapentin dose to increase available GABA in the brain, so we’ll see how that goes.
Please tell Chris Armstrong that ammonia IS DEFINITELY in over production with my Post EBV M.E. In fact I can prove it for him, and I can come to Melbourne if he needs me for any testing. When I exert myself I can produce so much ammonia in my urine that it looks like I have poured a bottle of ammonia disinfectant in the toilet bowl !!! Chris, if you read this, reach out. I am happy to help out with your research.
Isn’t that something! I notice my urine gets a lot darker…
Until I learnt to pace, my urine had such a high concentration of ammonia that I had to take pain killers (Paracetamol) after going to the toilet!
I too have ammonia in my urine and when I’d researched it, mentioned dehydration. However I drink mostly and plenty of water daily and have done so for many many years.
Do you mean like foamy urine? This has been happening on and off to me for years
Yes. So much froth that it doesn’t even flush away.
Wow! My doctors kept saying I was faking it and that I had too much toilet cleaner in the toilet.
I KNEW it was not right!!
Thank you
I have bubbles in mine and no one can tell me why.
I am utterly devastated by the fact that there is no, even small informal trial, to test this theory. Who will do this? OMF is not going to as they are committed to the other trials for the next 2 years! Are there other researchers interested? This really ought to get attention asap. I fear it will just slip back somewhere. In fact, it is imperative to try this, even in a small group of medically supervised people. Who will do this?
I didn’t mean to give that impression! It actually appears to be getting a lot of attention. Chris Armstrong with funding from the Amar Foundation is testing this hypothesis – and I have heard that Utah researchers are also looking into it. These video’s are from last year and early this year and I’m going to try and get an update on what’s going on. 🙂
Here’s the link to Chris Armstrong’s study – https://www.omf.ngo/itaconate-trap-study/
I think there was a further update on progress on one of the recent NiH webinars. Sounds like things are moving along.
Have you confirmed the state of play with the IDO try-trap hypothesis?
My understanding is that OMF quietly shelved it, after early negative kynurine trial experiments. There was also a Dutch LC pathology study, this year, showing *increased* IDO2 activity in brain cells.
Phair seemed to indicate it was dead, in a comment on Phoenix Rising, a couple years ago. Only Ron has indicated any intention to check further on it, in his Cambridge conference lecture this year.
Anyway. The IFNa Itaconate Shunt seems way more promising and wide reaching (beyond MEcfs)!
I’m looking forwards to your next parts. Especially if you can tie it in firmly with viral persistence (or partial reactivation), that seems to be a trending hypothesis. Does the shunt help explain the core pathology, even in this case?
From what I heard things were coming along – they had tested it in yeast, etc. – but I may have missed some things. I do remember that Dutch study but hadn’t looked into it.
I would think viral persistence would fit if it kept activating the innate immune system – which is driving the shunt. The next talk focuses on how the shunt could become chronic. I haven’t heard it yet – looking forward to it.
Yeah, the yeast experiments showed a tryptophan trap was biologically possible. A couple of years or so ago…
But OMF;s own update says: “Analysis of preliminary data for ME/CFS patients did not indicate significant disturbances in the Kynurenine pathway so further trials steps on hold.” https://www.omf.ngo/kynurenine-trial-in-me-cfs/
And I guess what I’m asking about viral persistence and the shunt is: can understanding the shunt lead to a cure, if viral persistence is the prime mover? Or only a partially effective treatment? Would resources be better invested in virus hunting/clearing, if so?
That’s a very good point about viral persistence.
As you are probably aware, Long Covid, etc often causes Epstein Barr virus reactivation. My theory is if you can prevent Epstein Barr virus from replication you can address the CAUSE and CONTINUATION of M.E.
Yes, and also other viruses that are turning up in ME/CFS patients, like Enteroviruses.
Dr Nancy Klimas recently said around 80% of patients were showing high elevations of enteroviruses.
Dr Klimas also recently talked about doing a trial of Interferon due to some patients temporarily improving during an infection.
I myself once had a near 100% week long recovery during a head cold. It was like being given my pre ME/CFS energy back. Possibly due to my body producing interferon and halting replication of enteroviruses. Unfortunately 24 hours after the last day of that head cold, I was bedridden again.
But that taught me this disease is reversible.
Elsewhere in Long Covid research there’s been suggestion if it’s not persistent SARS-CoV-2 or it’s fragments in reservoirs causing chronic symptoms, it could well be enteroviruses in the lining of the gut. And when you think about that, those enteroviruses have to be replicating because the gut lining is constantly replacing itself with new cells, which then become infected too
I think enteroviruses were suggested in the recent ‘low serotonin’ gut viral reservoir paper too.
Sadly those in a position to fund biological research didn’t do so because of the incorrect belief that ME/CFS was a behavioural disorder, due to corruption high
up in the psychiatric community with their fraudulent GET and CBT trial.
Those psychiatrists prevented so much biomedical research, that we could have had a treatment for ME/CFS years ago… and that could well have been a treatment ready and waiting for Long Covid patients.
Unfortunately that’s what happens when psychiatry hijacks a physical disease. They also block funding for biological research by pilfering funding for themselves.
Hello, thanks for the info, there is a treatement for this hipothesis that could be usefull for the patients ?
Hi, could you provide a link to “the recent ‘low serotonin’ gut viral reservoir paper”? I’m interested in reading it. Thanks!
Thanks for the update, Richard :). At least with long COVID we are getting a good look at viral persistence.
Hi Cort,
while watching part 2, the viral persistence hypothesis kept coming into my head. it sounds like a reservoir could very much be the “river” polluting the “ocean”!
After learning about the IDO metabolic trap, I experimented on myself with herbs and a supplement to try to influence it. This helped some and enabled me to move on to try other treatments.
I have had a preference for eating more protein than most for over 60 years. I am 70 years old. Testing done on me by an alternative MD in 2008 and 2010 showed a very dysfunctional krebs cycle in me. That doctor’s treatments did not help. That second test showed even weirder values for many metabolites.
My sources for choosing what to try for influencing IDO are the writings of the herbalist Shephen Herrod Buhner and the Canadian MD Abram Hoffer.
My method for choosing dosing is Muscle Testing also referred to as Autonomic Nervous System Testing. I tested each time because what and how much is needed is constantly changing.
The substances that I used are: Chinese Skullcap (Scutellararia
baicalensis, the skullcap that is more commenly available in USA does not work), Crinim latifilium, Isatis (Isatis indigotica), Japanese Knotweed (Polygonum cuspidated), and niacinamide. I took Isatis as a liquid tincture in organic cane alcohol. I took all other herbs as powders “brewed” in hot water. I took the niacinamide as a powder with no additives. As time went on, I gradually ate more carbs without negative effects.
I am no longer taking this remedy. This remedy helped me with energy, both physical and mental. I still have energy limitations normal people do not have. This remedy did not help me with orthostatic intolerance nor pain nor sleep. It might have helped some with reactions to substances.
I am still very debilitated. I took three breaks while writing this. Other things have helped me, none came from conventional medicine.
That is the thing, people talk about the Metabolic Trap like it was proven, factual science when nearly every finding (while limited by preliminary nature and small cohorts) suggested that it was invalid. Increased IDO2 being only one of many.
How does it tie in with fatigued t cells? I feel like this is a key piece of information to protect us from further harm, to restore our immune function.
I’m no fan of these hypotheses at all. If your hypothesis is based on measurements with extremely weak evidence (one experiment, never replicated) and you try to explain a not understood disease (ME/CFS) via a mechanism that isn’t understood at all (itaconate shunt) there’s far more tangible methods to deal with the problem.
I think it would make sense to start by replicating Armstrongs measurements. Then you either obtain something tangible even if the theory is incorrect, you have something you can actually build a theory on or you see that there are no irregularities.
Like this I see this ending up exactly like the IDO metabolic trap.
Karl, You could, of course, be right about the ultimate fate of the itaconate shunt hypothesis. At its best, science advances because of the inherent feedback between theory and experiment. One feature of the itaconate shunt hypothesis that needs emphasis is, as Ron Davis has described it, “ME/CFS may be a cell-autonomous disease.” This means only a fraction of patient cells (say, 15%) are sick. This makes strong experimental data difficult to obtain because any disease signature in blood will be diluted by the 85% of cells that are healthy. No one is more aware than I am that cell-autonomous theories are difficult to test, but cell-autonomous theories do have the advantage that they easily explain why it has been so difficult to discover an ME/CFS biomarker. The itaconate shunt hypothesis has good explanatory power, but it needs experimental testing/support. Multiple research groups, around the world, are working on just that.
Has anybody tried using Jak-stat inhibitors as mentioned by Rob Phair in some of his videos on the itaconate shunt https://youtu.be/PCnkkLlyVMk?si=A6cvZulJCrLFPLwh. Also DR Wes Ely Baricitnib https://youtu.be/GYi9NJelm2A?si=qqaUR_HFBd9E-LCA apparently about to commence trials albeit for long COVID would have implications for me/cfs Also does anyone know what Mark Davis [stanford uni]presentation at the recent me/cfs conference in Maryland was about?
I did. And I know a bunch of patients who took Filgotinib.
Some had some improvement 10-30%, other little/nothing.
– they had to be on it for a long time
I didn’t notice much of an effect.
Is filgotinib neccesary? I mean can we use other jak stat inhibitor like tofacitinib Or baricitinib?(only these 2 are available in my country)
I m not a Doctor, But as far as I understand, all these Jak-Stat inhibitors are similar, but their targeting is a bit different + selective.
You should find one that is at least jak-1 selective / inhibitive.
Filgotinib is chosen a think because less side efffects.
If you want to get prescribed / order please seek more advise
Adrian, Yes, filgotinib has been tried by multiple ME/CFS patients, but the drug has not been approved in North America, only in the EU and in Japan. Dr. K. De Meirleir, in Belgium, has trialed filgotinib in six severe ME patients and saw enough improvement that he recommends a larger trial. I have corresponded with one Australian patient who heard my talk on the Janet Show and went to Japan for filgotinib. After three days on filgotinib, his long-standing PEM was completely resolved. He was on multiple bioactive supplements at the time, so this is not well-controlled, but this patient remained free of PEM four months later. There are other JAK inhibitors among FDA-approved drugs, but I am unaware of patients taking them or of clinical trials to test them. Separately, a research group at the University of Utah identified FDA-approved drugs that are competitive inhibitors of CAD, the enzyme that catalyzes the first step of the itaconate shunt. One of these drugs was then shown to significantly decrease the probability of long-COVID (PASC) in a retrospective randomized clinical trial of COVID-19 patients taking or not taking this drug.
Thank you for your response and your efforts to cure me/cfs
Are you able to identify the drugs that are inhibitors of CAD ?
Regards Adrian.
these drugs are mentioned here starting min. 57 https://youtu.be/Y5AvIGvjyO4?feature=shared
Thanks Anna
Do you have any experience with these meds? I am also trying to access Filgotinib for my son who is seriously unwell but it’s not available in Australia so having difficulty
If anybody out there has any ideas I’d like to hear them.
It is very plausible idea to study ammonia in depth in ME/CFS. Maureen Hanson has pointed this our in her work and there are more suggestive leads:
1
Physiologically, accumulation of ammonia is one of the contributors to perception of fatigue (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3589241/). A unique population of dorsal root ganglia neurons has been discovered that specifically respond to low pH, ATP, and ammonia but not other painful stimuli (same paper). Also, ammonia levels physiologically rise after exercise – which „may contribute to the impaired voluntary activation of the motorneurons after prolonged and strenuous exercise” (https://pubmed.ncbi.nlm.nih.gov/15142684/ )
2
Ammonia is a strong neurotoxin with many secondary effects – which typically set in with a time delay of 8-12 hours. Ammonia effects include the disruption of the bood brain barrier (which means immigration of immune cells and activation of the CNS microglia). Ammonia also leads to astrocyte swelling and dysfunction (which can mean disrupted neurovascular coupling, brain edema, decreased cerebral blood flow, and thus CNS dysfuntion). Also, exposure of astrocytes to ammonia leads to Ca++ loading (which triggers glutamate release and thus excitotoxic damage).
3
It is compelling how much minimal hepatic encephalopathy (the cinical picture of early hepatic failure) and ME/CFS resemble each other: slowed mental processing, disturbances of attention and concentration, executive disabilities, psychomotor slowing and memory disorders. Unrefreshing sleep. Fatigue… Complex activities which require attention or information processing and psychomotor skills are mainly affected, such as driving a car or planning a trip…
4
In an old study, researchers have compared ammonia levels after experimental ischemia between alcohol abusers and ME/CFS patients. Guess who had higher levels? https://journals.sagepub.com/doi/10.1177/000456329403100507 . Would be good to replicate this study!
Unfortunatey the buildup of ammonia is hard to measure, but Armstrong et al. may find a way…
Any (experimental) way to reduce ammonia levels in our body?
Tauroursodeoxycholic acid (TUDCA) and sodium phenylbutyrate (PB) or both combined have been suggested to either scavenge ammonia (PB) or ameiorate its consequences
TUDCA: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4030606/pdf/gahmj.2014.017.pdf
Combined (proprint): ((https://www.biorxiv.org/content/10.1101/2022.05.02.490306v1.full ))
L-Ornithine may help
I wonder if the high ammonia levels could account for the poisoning feeling I had as did many others. I also developed Interstitial Cystitis early on. Apparently, excess ammonia has been demonstrated to destroy the gag layer of the bladder wall, as commonly seen in IC.
Same feeling here. (TBI, encephalitis, lyme and COVID)
Same feeling here ( Encephalitis, TBI, Lyme and Covid)
Poisonous feeling here too, though I’d wondered if that might be lactate (have not done lactate testing so far)
Since onset of mecfs I have had occasionally embarrassingly terrible smelling urine. Especially likely after any exertion. Wouldn’t there be a simple at home test for our ammonia output such as the ammonia test kits for fish tanks?
I suspect the fish tank tests may be in a different concentration range, but I’ll leave the answer to the experts – maybe ask Robert Phair directly by replying to one of his comments?
Does anyone else wake at weird hours of the night?
I wake at around 3 a.m. every night
No matter what time I go to bed ,it’s always
3 a.m.!!!
Yes, this happen to me at the same time nearly every night.
Whoa. This is the best explanation I’ve seen so far. I’m sure it’s no coincidence that the fatigue on my mild non-flareup days feels identical to the “oh crap im getting a cold” feeling from before I got cfs. Additionally, I have always thought of PEM as a build up of something in the body that’s not supposed to be there, which could definitely be ammonia or another byproduct of a broken krebs cycle. LDN has reduced my PEM duration significantly (from 3 days to a couple hours) and I think it may be making the cleanup process faster (it releases endorphins overnight which enhances the natural body healing processes, supporting the theory that PEM is some sort of acute damage).
As an aside, I enjoy when functions can be explained in a way that my classmates can understand easily, it makes it so much easier. “Remember ‘mitochondria are the powerhouse of the cell?'” and “so y’know back in high school when we did that really annoying unit about the krebs cycle?” immediately communicates the severity of cfs and the symptoms. Plus, it’s nice to have a comeback when people say the “you haven’t felt tired until you have kids” bs, “you haven’t felt tired until your cells stopped making over half of the required energy” clearly wins lol
Fascinating. Fingers crossed that compelling theories like this bear fruit, and soon
Is there any way to nudge the metabolism towards burning fat via carb limiting? I can tell i produce ketones via keto stix.
I’ve tried ornithine for ammonia toxicity but it didn’t seem to help any symptoms. Also, a serum ammonia test was normal. But I don’t know if that test is sensitive enough. Hard to believe that common tests couldn’t test such a huge disruption in the Krebs cycle..if true. Also, what of lactic acid?
Do diabetes patients also suffer from ammonia accumulation? You can smell this in your breath odor. Can switching to an emergency energy supply of our body explain the fact that glucose is not absorbed but is excreted by the body. You can measure this in your urine and smell the sweet smell. There are also patients who suffer from this.
Can anyone explain this?
Metabolic disorders are so complex and often difficult to treat. Then ME/CFS would be an acquired metabolic disease.
I have a lot of brain fog today, boxing day, but a light bulb went on with the mention of sweet smelling urine. Ketones spill over in diabetics when the insulin/pancreas can’t keep up breaking down glucose.
Interesting though, I’ve never tested even with pre-diabetes. Next time I’ll test my urine for glucose levels.
This sounds like it lines up with a previous theory I came across that said PEM is a result of toxins produced by anaerobic metabolism. However if I remember correctly, the toxin indicated was lactic acid (instead of ammonia).
Interestingly, I think between the two theories there is a reversal of “cause and effect”. The old theory assumed anaerobic metabolism is a result of hypoxia and blamed either the blood or the vascular system. This theory says the opposite — plenty of oxygen is reaching the tissues, but the cells simply aren’t using it!
That would explain why studies have shown ME/CFS patients to have abnormally high levels of oxygen in their veins after exercise.
i’m excited to see this theory get tested. I used to be a Grade-A student, but since my ME worsened it takes me nearly 30 minutes just to write and proofread a comment like this. Thank you so much to everyone who is working towards a cure. <3
I remain with the old theory – Why I say this – I have all the pathology tests to support the hypothesis.
So can we have high levels of cellular glutamate and low levels of glutamate in detectable plasma samples? Or would this hypothesis mean the patient would have high levels of glutamate in plasma? Didn’t Chris Armstrong find low levels of glutamate in ME patients? I also recall there was research that showed high levels.
Hi Cort, in end-of-year crash here… is it possible to add The Gist? I hope the reason there’s no Gist in this blog is not you’re in end-of-year crash too! I always really appreciate the Gist boxes!
Whoops! I forgot to put it in there – it’s in there now. 🙂
That’s great – thanks!
I’m hoping Cort, Robert or Chris can answer this for me:
Based on this hypothesis and existing metabolic research, would we expect to see plasma glutamate high or low if it is high intracellularly? I would assume it would be low in plasma if we are to assume there is a high and constant demand/utilization of it, and an accumulation of it in the cells? Or am I off base on that understanding?
Would we also then expect to see low levels of glutamine present in plasma, as it is being used rapidly to produce more glutamate? Also, is it correct that if glutamate is accumulating in the cells, and there is a lack of ATP, then glutamate and ammonia are not going to be broken down efficiently to produce glutamine?
And finally, would this high cellular glutamate also likely be present in brain cells (astrocytes)? And would this result in decreased production levels of glutamine in the brain? Meaning the brain is not able to efficiently remove ammonia, resulting in neurological symptoms?
Thanks so much for your time and help in my understanding this.
I wonder if this might be of interest . I searched for “food sources of interferon” and found this
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7130854/
It’s written in scientificy language but has a list of suggested supplementation at the end .
Merry Christmas & Happy new Year to Cort and all the forum .
Chris .
I appreciate you pointing out that “the innate immune system can also be activated by stress, injuries, or environmental toxins” i.e. known non-infectious (co-) triggers of acquiring ME/CFS.
Interesting read. Thanks! ME/CFS 6 decades & Long Covid 4 years. A few cases of strep throat as a child & adult. Severely infected tonsils for decades (Dr. Didn’t want to remove them).
Severe hemorrhaging from
monthly cycle. (Until emergency Hysterectomy) Pre-diabetes & now, severe dizziness. nausea, & amber colored urine. These things worsen when the weather changes 9 months out of the year in Seattle. The heat and sun clear out a lot of symptoms but lack of energy persists. Hanging on for a cure!
Yes, I also noted that. Might explain the diversity. 🤔
The day I see Witney Dafoe, stand up healthy, presenting his own recovery, I will believe and have hope.
Might this have something to do with the fact that physical and mental energies improve when I take aminos, for instance, Pharma GABA or especially Phenibut HCL?
With ammonia being produced with CFS …. What about the ammonia they are adding to the water called Chloramine . It’s a poor disinfectant and I have seen biofilms on my sink drains from it .
What if there is a latent bacterial infection after antibiotic therapy very possible because antibiotic do not enter the cell?eg Chlamydia psittaci causes all of the above from kreb cycle, citric cycle, vasculitis – What about the damaged cell membrane – these are only part of the problem. How do I know – I have lived through this scenario. What is being realized in this research has not been listened to – from years ago.
Just happened to come across this Chinese study https://www.frontiersin.org/articles/10.3389/fnut.2021.658630/full “A Botanical Product Containing Cistanche and Ginkgo Extracts Potentially Improves Chronic Fatigue Syndrome Symptoms in Adults: A Randomized, Double-Blind, and Placebo-Controlled Study”. Authors seem to be “employees of Nutrilite Health Institute, a division of Amway” (i.e. study seems to be related to a supplement producer rather than university), but at a quick glance study looks well done to me and the authors candidly discuss limitations such as that they found a statistically significant but small effect size, concluding that “Such a small effect size might be due to the fluctuating nature of CFS and the limited time points used to assess CFS in the current study. Therefore, the efficacy of the product against CFS is promising but needs further validation.”
What caught my interest is that among the measures used for comparing the success of treatment are both blood lactic acid and blood ammonia levels! The study found that:
– “In the current RCT, the level of blood lactic acid in the high-dose group was 0.2 mmol/L less than that of placebo group (2.39 ± 0.33 vs. 2.59 ± 0.50 mmol/L), an 8% decrease at the end of the intervention. (…) .3 ± 0.29 mmol/L vs. exercise 1.5 ± 0.3 mmol/L) that caused significant fatigue (46). Furthermore, the change in lactic acid concentration was significantly associated with the effectiveness for CFS symptoms in both univariate and multivariate analyses.”, and:
– “the blood ammonia level in the intervention group (high dose) was 5.5 μmol/L less than in the placebo group. Interestingly, an increase of 10–20 μg/dL (5.8–11.4 μmol/L) blood ammonia was observed in the subjects involved in cycling at 80–100% ventilatory threshold for 15 min, and such an increase led to the onset of fatigue (49).”
Interesting, no?
I googled this because a relative of mine adores Gingko and credits it with curing her Covid post-Vac leg pain.
Thank you for giving a detailed explanation of your theory. I look forward to part 2. My question is since there is a problem with the glucose and fatty acid inputs… should those be avoided? I have spoken to many fellow ME and long Covid sufferers with PEM and brain fog who also now have Diabetes or the worsening of symptoms that are not helped with medications. Being unable to exercise of course makes it hard to balance blood sugars but I do find fasting makes my symptoms better. If the cells are unable to use the glucose then one would expect higher insulin resistance, more insulin production and weight gain and inability to lose weight for Diabetics with this disease.
Does not explain the measurable abnormalities in microvascular filtration, oxygen extraction, low CNS dopamine and norepinephrine metabolites, abnormal cardiac MIBG reuptake or loss of neurovascular coupling all seen in cohorts of CFS/ME.
If ammonia caused brain fog it would be quite easy to treat.
This also relies on multiple assumptions without much in the way of evidence – that there is indeed a process in the Krebs cycle that suppresses normal ATP production to starve pathogens seems physiological implausible.
Do we have solid evidence that any form of ammonia is elevated in CFS/ME?
I get concerned when people propagate yet another theory that ignores all other findings and works in a silo which people presume has more supporting evidence than is actually factual.
You do realize that there is not just one single metabolic change in MECFS right? This is only one of them.
You do realise that theories and preliminary findings in small studies without replication are not facts?
Why do you guys of all orgs talk about the pandemic in the past tense?
Hi, This exciting area would certainly explain a lot. I’m chasing some additional information and hoping someone can point me towards some resources.
The final paragraph starts “Note that the itaconate shunt is not a hypothesis – we know it happens in the early stages of an infection”. How do we know this happens? Is there a good resource with references that explains how we know this is happening?
Thanks for any help
You would have to look it up in PubMed or elsewhere. The blog was simply based on Robert Phair’s presentation.
This makes so much sense. Would the depletion of CoA (and also likely depletion of bicarbonate, due to lactic acidosis from the low energy) cause a rate limiting effect on the urea cycle? Given that the first step of the urea cycle requires both.
I’m 56 & bedridden since 32 with fibromyalgia,m.e who knows I see so much but so expensive,a woman from services came & said your die in bed. Great that’s all I need I’m suffering so bad I can’t concentrate normally it’s living hell. You aren’t able to smile be positive it’s impossible. The pain is beyond I feel it in my sleep. I was a workaholic & miss it so bad. This is not living I’m so restless I feel suicidal 24/7 but won’t do it I suffer it but now I want to self medicate as not getting help needed I beg & beg but gets no where. It’s cruel & a curse. I have it severe that feel it in sleep I beg it to leave me alone . Everything is so expensive no one will let you pay a £1 or so a week till paid .
How can we reverse the itaconate shunt? This would surely be a cure?