

Dr. Pridgen is using three drugs to fight two pathogens.
The Inevitable Combination Treatment Approach?
Chronic fatigue syndrome (ME/CFS), fibromyalgia (FM), and long COVID are complex diseases that affect many systems and can cause a remarkable number of symptoms. It’s certainly possible that someone will get to the core of the problem and introduce a drug that takes care of everything.
It seems more likely, though, that each of these diseases will require combination treatments. It was a combination of drugs, after all, that throttled HIV. Multiple drug combinations, each of which features at least two drugs from different classes, are now available.
Ditto with Hepatitis C where the addition of a second drug greatly boosted treatment effectiveness. Zepatier, for instance, combines a protease inhibitor (Grazoprevir) and an antiviral (Elbasvir). Ninety percent recovery rates are now being touted.
So what the heck are we doing with one drug therapy in long COVID (Paxlovid) or regarding herpesvirus infections in ME/CFS. At this point trying to knock out a complex disease with one drug seems like bringing a knife to a gunfight.
Indeed, concerning long COVID, David Putrino, the leader of the Mt Sinai Center, stated, “Everyone wants a single treatment; that’s not realistic for long COVID.” Bruce Patterson is using a CCR5 antagonist plus statins to try to knock down long COVID. Resia Pretorius had found that an unusually strong triple drug anti-platelet, anticoagulant approach is necessary to move the needle in long COVID.
Some ME/CFS researchers concur. Travis Craddock’s models at Nancy Klimas’s Institute for Neuroimmune Science, for instance, found that while using a herpesvirus antiviral by itself allowed herpesviruses to quickly rebound, adding rituximab caused the virus to flatline. Nancy Klimas is using two drugs in an attempt to knock out neuroinflammation first and then reset the HPA axis later, in Gulf War Illness and ME/CFS.
Given all this, it seems remarkable, in retrospect, that anyone would expect a one-drug approach (Valtrex, Famvir, Valcyte) to knock out the Epstein-Barr virus or other herpesviruses in ME/CFS. It’s perhaps not surprising that Pridgen has been able to get his duo antiviral drug approach in fibromyalgia almost to the finish line. (A phase 3 trial is waiting clearance from the FDA to start)
Early Paxlovid Long-COVID Reports Not Promising
While nothing has been published, anecdotal reports suggest that the Stanford Paxlovid (which is a two-drug combo study (nirmatrelvir and ritonavir)) – which has been completed – did not go well. (While Paxlovid is a two-drug combination, ritonavir – the second drug – does not interact with the virus itself. Instead, it stops the body from metabolizing the first drug (nirmatrelvir), thus boosting its potency. Paxlovid, then, is essentially a one-drug formulation.)
Meanwhile, a bunch of Paxlovid trials have gotten underway recently. A 900-person RECOVER Paxlovid invitation-only trial appears to have gotten underway in July, a 100-person Yale study started in April 2023, and a 400-person Karolinska study opened its doors in May 2023. We should know the results of several of these in 2024.
Time will tell how the Paxlovid trials end up, but all these duplicative trials (they seem mostly to be using the same dose) to me, point to the general disorganization, messiness, and inefficiency of our medical research system. Do we really need 4 Paxlovid clinical trials to tell if the drug is helpful or would it have been better to use our limited resources on testing other potential treatments?
Pridgen’s Combination Plus Long-COVID Trial
And here comes Dr. Pridgen, MD. He’s gone the furthest with the duo-drug synergistic approach in our neck of the woods. Not only was his two-drug antiviral therapy (valacyclovir/celexicob) aimed at a condition – fibromyalgia – that few people associated with viruses – but as was noted that approach has made it to Phase III trials.
When Bateman Horne Center tested the duo-drug approach in a small placebo-controlled, double-blinded Phase II long-COVID study, the results appeared to be pretty good. Pridgen reported that 14 weeks of the drugs resulted in “clinically and statistically significant improvements in fatigue, pain, and symptoms of autonomic dysfunction” and that the drug combination was well-tolerated; i.e. no significant side effects or adverse events. Thirty-seven of 39 participants completed the trial.
Now comes the “IMC-2 and Paxlovid Synergy Treating Long COVID Trial” featuring three drugs: the IMC-2 combination (valtrex/celecoxib) and Paxlovid.
Study Rationale
- Continuous use of the IMC-2 approach “provides daily suppression of herpesviruses such as VZV, HSV-1, HSV-2, EBV, CMV, HHV6, and potentially others”.
- Paxlovid is a combination antiviral that primarily uses nirmatrelvir – an antiviral that belongs to a family of 3C-like protease inhibitors developed in the late 2010s to fight the feline coronavirus. These 3C-like protease inhibitors block the activity of a viral enzyme called 3C-like protease (3CLpro).
- Knocking down the coronavirus helps reverse the immune changes (exhausted T and NK cells and B-cell activation) it caused, allowing the IMC-2 protocol to be more effective at knocking the activated herpesviruses down. Pridgen has found that he can use lower doses of his IMC-2 combination to the same effect than when it’s used alone.
In his office, Pridgen treated long-COVID patients with IMC-2 (2-dose every 12 hours (valacyclovir 750mg/celecoxib 200mg pill) for 120 days plus 1-pill of Nirmatrelvir/1-pill Ritonavir (every 12 h) given for 15 days. During the time the participants were taking Paxlovid the IMC-2 dose was cut in half.
Pridgen compared their results to 13 long-COVID patients treated with IMC-2 alone. (Note that Participants had to report that “they were substantially less healthy than before, suffered from severe or moderately severe fatigue, and at least moderately severe brain fog or palpitations (with dizziness & tachycardia) or both.
He used a five-point grading system (none, mild, moderate, moderately severe, severe) to assess fatigue, brain fog, and symptoms of dysautonomia. The primary endpoint of this prospective study was the % reduction in moderately severe and severe symptoms of fatigue.
Results
Pridgen found substantial reductions in fatigue, brain fog, and dysautonomia in the IMC-2/Paxlovid group which were significantly better than found in the IMC-2 only group.
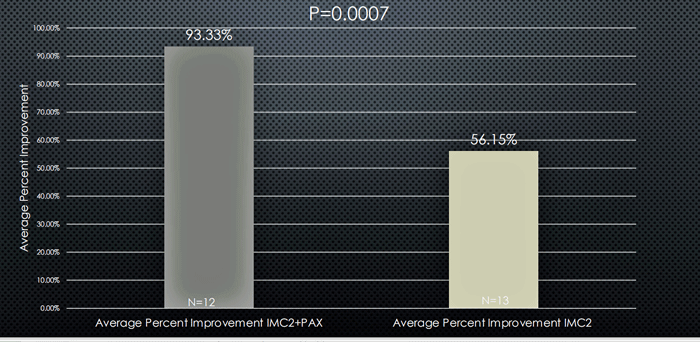
Fatigue results – Dark grey bar – IMC-2 + Paxlovid; light grey bar – IMC-2.
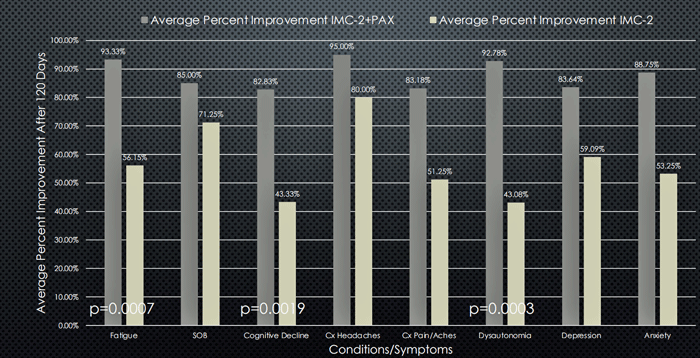
Dark grey bars – IMC-2 + Paxlovid; light grey bars – IMC-2.
Pridgen reported that when used with Paxlovid, he was able to cut the IMC-2 dose (750mg/celecoxib 200mg pill per side (12 h)) by half and achieve the same results. He also reported that the IMC-2/Paxlovid combination works much more quickly than IMC-2 alone. Pridgen reported that he’s treated about 50 long-COVID patients. While using just IMC-2 tended not to work in about 25% of long-COVID patients, the IMC-2/Paxlovid combination works in many more long-COVID patients. The only notable side effects were the metallic taste that Paxlovid produced which is well known and disappears when the drug is done.
Pridgen noted that with the free Paxlovid no longer being free in the U.S., in 2024, getting it will become more difficult.
Hope for Other Viral Triggers???
Pridgen reports that Paxlovid’s 3C-like protease (cysteine) formulation potentially has activity not just against the SARS-CoV-2 coronavirus but against many other viruses, including other coronaviruses (MERS and others), (picornaviruses (rhinoviruses, enteroviruses and others)), astroviruses (diarrhea), caliciviruses (gastroenteritis), hepeviruses (hepatitis including hepatitis A), alphaviruses (arthritis, encephalitis, rashes and fever), flaviviruses (West Nile virus, dengue virus, tick-borne encephalitis virus, yellow fever virus, Zika virus) and others.
We don’t know if Paxlovid is effective against these other viruses, but if it is, it could potentially be helpful – when applied with Pridgen’s IMC-2 protocol for herpesviruses – against other viral triggers in ME/CFS. There’s also the possibility that the viral trigger for some people with ME/CFS was another coronavirus, in particular, one that could be associated with the ACE-2 issue in ME/CFS.
Note that the study was “prospective” and was not placebo-controlled or double-blinded (the patients knew they were getting the medication). Pridgen has funding for a pilot placebo-controlled, double-blinded study and is beginning to look for a location to host the trial.
THE GIST
- Long COVID is often not just about the coronavirus – there’s also often a herpesvirus reactivation to worry about. If that’s true, then why are we trying to kill two birds with one stone (two viruses with one medication – Paxlovid)? The situation seems even more untenable given that combination drug therapy is needed to knock down HIV (3 drugs) and hepatitis B (two drugs) and other pathogens.
- Dr. Skip Pridgen brought the first duo antiviral approach to the herpesviruses in ME/CFS/FM with his IMC-2 protocol. As the penultimate phase-3 trials of that approach are taking place in fibromyalgia, Pridgen brought his herpesvirus-busting protocol to long COVID ( at the Bateman Horne Center) and reported that it worked: fatigue and other symptoms declined.
- After Pridgen, though, was unable to make progress in about 25% of his long COVID patients, he added Paxlovid to the mix and reported – in a small non-blinded, non-placebo-controlled prospective trial – some pretty darn impressive results. Fatigue scores, in particular, shot down dramatically and significant improvements were seen in brain fog and dysautonomia symptoms. Paxlovid plus his herpesvirus meds was far more effective than Paxlovid alone.
- With Pridgen reporting that Paxlovid might also be helpful against many other viruses, it’s quite speculative but some possibility this triple drug combination might be helpful to those with ME/CFS/FM and other illnesses who experience herpesvirus reactivations and other viral triggers.
- With those results in hand, Pridgen was able to raise funding for a proper trial and the search is on for a location. Pridgen’s duo antiviral approach was new for fibromyalgia and ME/CFS and his triple antiviral approach is new for long COVID but combination therapies are probably going to be needed in long COVID, ME/CFS, and similar diseases.
- Thanks to the many people who have supported Health Rising thus far in its end-of-the-year drive!
Applying The Arseneau “Should I Try a Treatment or Not” Test
The Arseneau test assesses the factors below to help decide whether or not to try a treatment. Note that different people will get different results. For instance, people with more resources may feel more comfortable trying more expensive and unproven treatments. Likewise, people who’ve had bad reactions to treatments in the past may be less likely to try things that don’t have a strong evidence base. In other words, the final results are person-dependent.
- The credibility of the source – While Dr. Pridgen has always been quite optimistic about his approach, for me, he is a good source. He has, after all, been able to shepherd an antiviral treatment for fibromyalgia (of all diseases), to the point where a phase III trial is awaiting clearance from the FDA. That really says something.
- Quality of the evidence – lacking. Small study and good results but no placebo controls or blinding.
- The benefit, the cost, and the risk–benefit analysis – the benefit appears to potentially be quite high, the cost is probably high, particularly in 2024, and since several studies have assessed the risk of IMC-2 and Paxlovid, the risk appears to be low.
Arseneau Test Conclusion – A possibly costly drug combination not covered by insurance in a small, preliminary trial with some very good preliminary results probably puts it out of the range for many of us but since it appears to be safe, others may want to give it a shot (if they can find a practitioner.) If Pridgen can get a trial going – and there’s no reason to think he shouldn’t be able to – we could have a better sense of efficacy by sometime next year.
Conclusion
Facing a two-pathogen situation (the coronavirus and herpesvirus reactivation) Pridgen took his combination antiviral approach to the next level. We’re still at the very early stages but thankfully Pridgen has the funds to do what the ME/CFS field has so rarely been able to do – test out his new protocol – and we should learn in the not-to-distant future how it went. Long COVID patients, of course, will want to know but while Paxlovid was specifically created to battle the coronavirus, the possibility that it might help against other pathogens makes the trial of interest to people with ME/CFS and/or fibromyalgia.
Overall, Pridgen’s approach and others that feature combination therapies are a good sign that the long COVID and ME/CFS/FM fields are coming to grips a bit with the complexity of these diseases.
Health Rising’s Donation Drive Update

With 10 or so blogs under its belt, Health Rising has been providing updates on Dr. Pridgen’s work for over ten years. Why? Drugs don’t come easy for ME/CFS or fibromyalgia and when they occur we jump on them and now that long COVID is in the mix we’re jumping on it too.
If keeping up to date with new treatments is something you’re committed to please support Health Rising in a manner that works for you.






Please provide a “Gist” for all your articles.
Some of us have so much difficulty focusing with our disease.
This would really help.
Thanks.
For the second time in three blogs I did it and forgot to put it in. It is in now 🙂
Thanks, Cort. Without these summaries, I am totally unable to comprehend all the details in the long articles.
Can’t help but wonder if missile blew might exasperate restless leg syndrome given that it can increase serotonin. Anyone have any insight?
I totally agree with Kristi Hanna. I am having a hard time understanding this blog. Does Nancy Klimas have the golden treatment???? I’m a past patient and would like to know if this is something to push for.
Nobody that I know of has a golden treatment.
Dr. Klimas has a combination treatment employing etanercept and mifepristone that she has been testing in Gulf War Illness and received funding to try in ME/CFS. I don’t have results for either one. The GIST is in.
Ik heb het gevoel dat wij de ernstige bedlegerige ME patiënten ontzettend achterlopen op long covid waar nu zoveel tijd en geld wordt ingezet. Het lijkt wel of nu de diagnose ‘ virusinfecties ‘ op alles geplakt wordt. Hoe komen wij die op bed liggen aan wat voor diagnose dan ook, als er nog niet eens een bloedtest wordt aangeboden om te kijken of er überhaupt wel een virus of bacterie in het spel is !
English Translation:
I have the feeling that we, the severe bedridden ME patients, are very much behind long covid where so much time and money is now being used. It seems as if the diagnosis of ‘virus infections’ is now being stuck on everything. How do we who are lying on the bed get any kind of diagnosis, if not even a blood test is offered to see if there is a virus or bacteria involved at all!
ja, dat is waar! En we zijn zo heterogeen en zo een complexe ziekte..yes, it is true.! And we are so heterogenous and it is such a complex desease
Yes, Lenny I must wholeheartedly agree with you. ME is miles behind long COVID regarding research funding and clinical acceptance I am sure. Our hope is that the long COVID research and treatment efforts will carry over to
I think this is not totally true because Long Covid is considered as a psychosomatic illness.
I have been diagnosed with chronic lyme , found two years after symptoms. The test was positive. In the years following, I was diagnosed with reactivated Epstein Barr and chronic fatigue. This year, I came down with covid and didn’t get better with 5 days of antivirals, so now I have long covid. I will not go into all the medications, supplements, and treatments I have tried.
I am so happy that testing is going forward for long covid that may spill over to help ME/CFS. The one concern I have is that finding a doctor who will prescribe off label drugs is a nightmare. They are afraid of losing their medical license, at least here in the Midwest. It really doesn’t help when you have potential drugs that could help but you can’t find a physician that will take the risk. I know because I’ve been there or are there.
I do find hope that studies are being done on long covid because if you have any other conditions that I have listed, medical research has mostly ignored them.
In some ways you are in good shape (lol) because with long COVID you now have a condition which, if the clinical trial results are good, doctors should be willing to treat. Some drugs are being tested now in long COVID that we’ve hoped for years would get tested in ME/CFS.
Very true
Thanks Cort
I hope that you and everyone else suffering from chronic infections or infections that come and go and come back again, whether viral or bacterial, have insisted that your doctors order a serum gamma globulin test for IgG, IgM and IgA. For 27 years I lived the medical odyssey so many of you know so well: doctor after doctor ran the same basic labs and told me I was fine when I was too sick to hold a job, often to sick to brush my teeth. Finally, an amazing woman named Kristin Loomis (founder and head of the HHV-6 Foundation), suggested I get my gamma globulin levels checked. It’s a simple blood test. A few days later I learned I have a primary immune deficiency known as Common Variable Immune Deficiency (CVID), a very UN-common disorder I now know I inherited from my mother. It’s a complex disorder — one of about 400 kinds of similar disorders — but the upshot is that I can’t make enough antibodies to fight almost any kind of infection. There is treatment and, although it’s not a complete solution, it helps a great deal. Whenever I read a comment on Health Rising from someone who has multiple chronic infections, I wonder if they, too, have some sort of primary immunodeficiency. All it takes is a simple blood test to find out, but for some reason doctors rarely order it. Maybe that’s because the disease is rare and they’re thinking hoofbeats and horses; maybe it’s because so many of us have symptoms that don’t fit the expected template. Whatever the reason, given the years of suffering and the missed opportunities so many of us have experienced, it sure as hell seems worth throwing a small fit during your next doctor appointment if that’s what it takes to get the blood test done. I wish you all improved health and a peaceful 2024.
Thank you for this information. I have never heard of this type of test. I do have a functional medicine doctor that would probably be ok with the idea. I have seen numerous doctors and spent tons of money. I definitely will look into this for sure. Thank you again!
I hope you’ll let us know if you learn anything interesting or useful. I wish you the best.
I’m not sure what this blog is actually about. For one thing, Dr. Nancy Klimas’s Institute hasn’t used rituximab in about 8-10 years. I was one of the few that tried it. It didn’t help at all.
The blog is about Dr. Pridgen’s triple combo antiviral treatment for long COVID and its possible potential implications for ME/CFS/FM. The GIST is now available to read.
The Rituximab part referred to a model Craddock used to assess combining an antiviral with a monoclonal antibody (Rituximab). It wasn’t intended to promote Rituximab – rather it was intended to demonstrate how much better combination treatments can have with viruses.
They always talk about fatigue, but what about pem? If you have severe pem you can’t live your life. What about effects on pem?!!!
That was an attempt to modify ones immune system. My treatment approach is to fix the underlying problem. Cort did a good job of suggesting other avenues that could address similar or related conditions.
Cort, thank you for covering this.
I just wanted to quickly share to you for awareness – the lead report on KSTP’s Channel 5 News Minneapolis – St. Paul (ABC News affiliate) broadcast recently (12/17/23) on ME/CFS & LC.
https://kstp.com/kstp-news/top-news/no-treatment-no-cure-patients-await-answers-about-debilitating-disease-related-to-long-covid/
KSTP: “No treatment, no cure: Patients await answers about debilitating disease related to long COVID” (video included)
ME/CFS “has been relegated to the margins of medicine when you don’t have money and funding allocated for it,” he added.
“Earlier this year, the Minnesota Legislature invested an ongoing $3.146 million a year to address the post-COVID illnesses that have affected about 12% of Minnesotans since 2020, she said.”
Just sharing your way, as leading off an evening broadcast seemed to be pretty impressive exposure.
(apologies if you already saw or was shared)
Dakota
Thanks, Dakota! Really good to see this for ME/CFS and long COVID!
ME/CFS “has been relegated to the margins of medicine when you don’t have money and funding allocated for it,” he added.
“Earlier this year, the Minnesota Legislature invested an ongoing $3.146 million a year to address the post-COVID illnesses that have affected about 12% of Minnesotans since 2020, she said.”
Hi Cort,
Nice summary, as always:-) Do you know when the 15 days of Paxlovid was given? At the beginning of the 120 days or at the end? I take Celebrex now and used to take Valtrex (I was a Dr. Lerner patient the last few years of his life), but never took them together. I think I can get my Dr at NOVA to try this for me. Thanks for doing all these summaries!
If I have this right Paxlovid is designed to get at the coronavirus – which has dysregulated the immune system. Dr. Pridgen gave the long COVID patients 15 days of Paxlovid and 120 days of his antiviral protocol.
That is IMC-2 (2-dose every 12 hours (valacyclovir 750mg/celecoxib 200mg pill) for 120 days plus 1-pill of Nirmatrelvir/1-pill Ritonavir (every 12 h) given for 15 days. During the time the participants were taking Paxlovid the IMC-2 dose was cut in half.
Note, though, that after the end of the year Paxlovid is going to be harder to get.
Hopefully, some of the Paxlovid trials are following the changes wrought by the drug in detail. That way we might be able to see what was still “out” in the immune system.
Normal dosage of Paxlovid is *2* pills of Nirmatrelvir + 1 pill of Ritonavir every 12 h. So this regimen is using a reduced dose?
Vlad, What a good catch about the dose! Did you ever find out if Pridgen used the normal dose of *2* pills of Nirmatrelvir + 1 pill of Ritonavir every 12 h–or did he reduce the nirmatrelvir to one pill?
We gave it as early as possible. It took 2-6 weeks to accumulate the necessary 15 days. The sooner the better, as it seemed to reset the immune system and speed up the recovery.
Thank you so for your efforts! My question is whether any patients suffered viral rebound after their 120 days. I had Paxlovid rebound after I went off the 5-day course. And my HSV1 load is so high that I have a terrible rebound and series of breakouts each time I have completed a prescribed antiviral course. The higher the dose, the worse the rebound. I also remember people in a Facebook group trying the original Famvir/Celebrex having rebound each time they went off, and saying that, if it worked, they would have to stay on it the rest of their lives.
The drug companies LOVE these combinations of easy to use, well-known drugs which are OUT OF PATENT.
Instead of focusing on how much of X, Y, and Z drugs, the doctors can now prescribe such things as (much more expensive and under new patent protection) a combination of 2X+3Y+Z – and SWITCH prescriptions every time they want a different combination.
They need to leave these alone, figure out WHICH components do what, do a PROPER EXPERIMENTAL DESIGN (yup, statistics) to DETERMINE, possibly for EACH patient, where on the combinatorial landscape (aX+bY+cZ) they need to be.
I know a lot of new drugs are supported by expensive research – that is somewhat inevitable under our winner-take-all system for new patents – but this is a racket. An expensive racket. Because the drugs are NOT new, and there is a lot of research already done on them, clinical research – just not in these precise combinations.
As a grad student in Nuclear Engineering, I learned how to do these designs – and how to interpret results – from my now-husband. It’s the EASY way to find cross-effects and synergistic benefits. It produced STANDARD easy-to-interpret 3D graphs back in 1970 on primitive mainframes.*
Must the wheel be reinvented every single time? And these scientists and companies should KNOW this stuff.
*Forgive me if, after all that schooling, and ten years work, 34 years of ME/CFS has made some of the details fuzzy. I used to have a brain.
Cort, totally agree – why do we need four Paxlovid trials?? What a waste of resources. I eagerly await Pridgen’s results. Thanks for sharing this.
Huge thanks to Dr. Pridgen for your ongoing work on behalf of those of us with fibromyalgia and ME/CFS!
And, as always, Cort, thank you for keeping us informed and giving us hope in this long darkness.
Could I assume that “mycoplasma pneumonia” would also be one of the various reactivated virus’ considered in this study?
I see that included only sometimes (as it is in this very interesting study using nebulizer antioxidant/anti-pathogen). I have a difficult time deciphering if the numerous antivirals used in all these trials are similar.
https://www.sciencedirect.com/science/article/pii/S2666354623001345
“Identification of CD8 T-cell dysfunction associated with symptoms in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and Long COVID and treatment with a nebulized antioxidant/anti-pathogen agent in a retrospective case series”
“As many different persistent pathogens have been associated with ME/CFS as potentially causative or contributing to disease via reactivation, viruses such as EBV, CMV, HHV-6, HHV-7, HHV-8, (Unger et al., 2017) (Buchwald et al., 1992; Ablashi et al., 2000), human parvovirus B19 (B19V), enteroviruses (O’Neal and Hanson, 2021; Chia and Chia, 2008), lentivirus, and bacteria (Beyond Myalgic Encephalomyelitis, 2015) such as mycoplasma, lyme and Q-fever, this nebulized agent could potentially help dampen pathogen load of multiple infectious agents at the same time. This nebulized treatment also has multiple ingredients capable of acting as immune modulators through their ability to attenuate NF-κB signaling, a process essential for signaling through the TCR, thus decreasing over-activation and potentially averting subsequent dysfunctional CD8 T-cells”
“1,8-cineole, β-caryophyllene and methylcobalamin each have antiviral properties to SARS-CoV-2 [86–88). β-caryophyllene has broad spectrum antibacterial properties (in vitro) against several bacterial species, including S. aureus, K. pneumoniae, and P. aeruginosa, and in vivo for M. pneumoniae(Dahham et al., 2015). β-caryophyllene also has broad spectrum antifungal properties against several fungal species”
thank you Noelyne. This is really interesting and fits right in the connection with pneumonia and ME that I’ve been looking into lately. I will read it more carefully later
Is there any information on the patients in the weeks after the protocol was completed? Will patients need to continue with the medications in order to continue to manage symptoms? Also, if these medications get approved to combat long Covid, will all the patients with FM and ME be left out in the cold until another whole study that will take years to complete is done?
I think Paxlovid is next to useless. In August, my husband and I got Covid at a national conference. We immediately began to take Paxlovid. In a week or so, we were testing negative for Covid. But, in one month, I began to experience episodes of A-Fib and high blood pressure. In a complete heart workup the year prior, I had none of these issues and no history of this in my family at all. A visit to PubMed, the online portal to the National Library of Medicine had reports of exactly the kind of problems I had started having at one month past Covid. I can’t do anything now compared to before Covid without rapid and skipped heartbeats, a possible precursor to a heart attack or stroke. My ND said some of his patients who took Paxlovid had Covid rebound.
There seems to be some very basic questions that remain unanswered from years ago. Why is everything now in the Long Coronavirus camp?
Jennifer, I agree with you. I have had ME/CFS since 1984 and maybe that is why I was more severely affected by Covid. I think Covid is getting more attention because millions have died from it and even Long Covid can have life-threatening symptoms. I don’t, however, believe that there are any treatments that have been universally successful in treating Long Covid. My doctor treats both ME/CFS and Long Covid and while his treatments are helpful, they are no cure for either condition.
This is a sensible approach and I hope there will be more effort on this front as time progresses. However, what a lot of patients have been advocating for also appears to be very random, without accounting for any pharmacological knowledge.
I certainly don’t understand the incentive to try one pill of Paxlovid every 12 hours for 15 days, that certainly doesn’t appear to match any pharmacological data (see also the recent https://www.biorxiv.org/content/10.1101/2023.12.20.572655v1#:~:text=The%20persistence%20of%20an%20intermediary,with%20a%20polymerase%20inhibitor%2C%20remdesivir). Currently there also isn’t too much evidence of LC involving exhausted T and NK cells and B-cell activation (one could argue that there is some evidence of exhausted TEMRA T-cells in LC, but B-cell activation or exhausted NK cells hasn’t been observed, beyond some possibly opportunistic infections like EBV).
The kitchen sink approach works in HIV/AIDS because one understand a lot about what is going on what is necessary, not because a lot is thrown at the problem. If viral persistence was indeed a driver of LC and/or ME/CFS then I agree that a combination therapy likely seems necessary. One of the problems is establishing safety for single drugs first, that is one of the things the current LC trials are doing (without that combination trials can’t exist). Naturally most researchers want to trial combination therapies for longer durations, but without safety data for the single interventions one cannot get there. Stanford was desperate to include a 30-day Paxlovid arm and wasn’t even granted that. It’s a hard fight, especially with the pharmacological industry and when it comes to combination trials without safety data or markers, it’s fight that cannot be won. Even in HIV/AIDS when markers were established and therapeutics had been developed and shown to be safe as well as effective it required significant efforts to convince the industry of combination therapies.
I understand the critique at there being 4 different Paxlovid trials and I agree that coordination should have been far better, but one should also note that these trials are all very different trials by design. The Stanford trial was the first rigorous trial ever conducted on LC, so it served as a starting point to learn about cohort selection and trial design, the Yale study is looking at immunological changes during Paxlovid, whilst RECOVER is also testing Walt’s simoa assay as part of the study as well as changes in blood and even feces. I can think of many things that are far less sensible than doing multiple trials with the only drug that had an efficacy against SARS-COV-2 at the time when these trials were designed. I do think that RECOVER should desperately open up an adaptive platform trial.
This treatment is very toxic. I would say: be careful. There are no objective points at this moment to take this medication.
I’ve been dealing with ME/Fibromyalgia (I don’t the difference because I deal with symptoms of both) since 2010. It was horrible for the first 5 years and is better off and on.
What I’m confused about (lol, now everything is confusing to me), is the role that the herpes and Epstein Barr viruses play in relation to Long Covid, FM & ME.
I suffer from an ongoing herpes infection in my nose. I’m on a daily preventative dose of acylovir (.5 gm) and have the active dose (2 gm twice daily for 2 days) on hand always. But my ongoing infection rarely is completely gone and I don’t like to over use anti-virals, so I deal with an ongoing infection. It’s better than before I started the preventative dose of acylovir, but it never goes away.
I recently had my EB antibodies checked and the antibodies were 687! I don’t remember having Epstein Barr my antibody levels suggest I’m still actively fighting the disease.
I also have 4 other autoimmune diseases, including on that attacks the roots of my teeth, which is so awful because I have no dental insurance.
I’m completely lost and have no idea what to do. I feel like until I can get the herpes virus under control and do something about Epstein Barr, I’m never going to get my energy back.
Any insight? Anyone have any suggestions where I should begin? I’ve been trying to get a handle on all of this but, as we all know, doctors have no idea what to do with me. Mostly they just tell me that despite all of my autoimmune conditions and my ongoing active herpes and my EB antibodies, I’m fine **eye roll**
I have breakouts every other week during flu season and in response to every single stressor ( mental or physical) . Vit C (2g) , Lysine (3g) , black seed oil ( 1 tsp ) orally and vit C+ lysine topical mixture ( made in water) and applying around nose area twice a day eases the pain/ discomfort and reduces recovery time for me from 5-7 days to 2 days. Someone recommended this on a phoenix rising blog in 2010, I have used it ever since and it has helped me stay off antivirals. When its quite worse , I just double down on this dose for the first day.
Thank you Sum! Honestly I have so many things to take care of around all of my conditions, the most tedious being plaque psoriasis, that I tend to just use medication for the things I can. I admire your approach!
Upon looking up celecoxib, it doesn’t appear to be an antiviral as is described in this article. Can anyone help clear this up for me? The rational for its use is that it assists in preventing viral replication though it appears to me to be a pain killer and not an antiviral med. Any explanation on this would be greatly appreciated.
It blocks Cox1 and Cox2 enzymes and viruses use both to make new viruses.
Skip, I hadn’t heard that about Cox1 and Cox2 inhibitors. My information is that they just treat inflammation. Interesting.
Email me at tsasurgery@gmail
And I will send you some links.
I think you’re talking about celebrex, right? That’s actually an antinflammatory. They also marketing as a pain reliever, but that’s just because it treats inflammation which is a cause of pain.
I know alot about celebrex because a friend worked for Merck and they had a competing drug called vioxx which had the same mechanism as celebrex, but Pfzier, the makers of celebrex withheld information about the effect on the stomach, which Merck made public, so Vioxx was banned, but you can still get Celebrex.
Regardless I can’t take any anti inflammatory medication because I have a blood clotting disorder and will be on blood thinners for the rest of my life. It makes it really difficult. I can never take anything except steroids to reduce inflammation and they make me crazy!
My doctor has prescribed me acyclovir & valacyclovir because of EBV & VZV reactivation after Covid. I am severely affected and it is my last hope for recovery. Unfortunately, I get severe nausea and abdominal cramps after just 2 days. If anyone has any tips on what I can do to tolerate it better, I would be really grateful.
Hi Lisa, stay strong it is not your last hope. Even without this drug you could be much better with time. Many people will be better. Pace yourself is my advise! I know it is difficult but time will learn you. Take vitamin, pace yourself, meditate and take probiotics. Drink water, no sugar. Be patient. Best wishes!
to me, this treatment is nothing else than symptom treatment. We are all infected by many viruses of which many persist in our bodies, but are kept silent because of our immune system. The goals should thus be to understand how to improve one’s immune system.
I experienced a wonderful week and a half improvement after starting Paxlovid. I thought I was on my way to healed but it was short-lived. This was the first clue my illness might have a viral component. Since then I have been asking and searching for a way to take Paxlovid again, but haven’t found a doc willing to prescribe. I understand there are risks, and I’m willing to accept them. Does anyone know how to find a doctor to prescribe? Or how to get involved in Dr. Pridgen’s study? p.s. There’s an organizing effort of people with experiences like mine contacting Pfizer to try to get them to look into this.
Thanks, Cort, for the amazing article. I don’t know how I missed it earlier!
I felt amazing after taking paxlovid also. I got Covid for the second time in September last year and was prescribed antivirals. I quickly recovered from Covid and tested negative but also felt better than I had in years – pots symptoms greatly reduced, energy, heat tolerance, clear thinking, clear sight, no dizziness – it was amazing while it lasted. 2-3 weeks.
Trying to find out more about what this means for me.
I noticed that in the comment called the gist, HIV was mentioned. I almost hate to ask, but is that now part of the treatment for people who have persistent problems with covid? I’m asking because I’ve had some pretty serious problems, and I really don’t see HIV treatment in my future.
I don’t see any reason why that would be so. Very different viruses! I wouldn’t worry about HIV.
I guess it is just poorly written. I think the author might have meant that “if HIV is an issue,” it would involve a more complex cocktail. Thanks!
Thanks for the article! What is the source of your info and charts? I’m missing the link to the study “IMC-2 and Paxlovid Synergy Treating Long COVID Trial”. Thank you!
Hello !
We are in 2025 now the 18 August .
The results of Imc-2 + Paxlovid today is good treatments now for today?
Or others mixed?
Thanks!!
Joël