

This study took exercise studies to the next level by studying the effects of exercise on the muscles.
One of the dangers facing the long-COVID research field was an over-emphasis on immune functioning and an under-emphasis on metabolism and energy production. While exercise studies showed up pretty early in chronic fatigue syndrome (ME/CFS), it took the field a while to incorporate them. Indeed, some of the findings were so striking that they were largely discarded by outside exercise physiologists.
The findings from Systrom’s recent invasive exercise studies and Naviaux’s and others’ early metabolic studies helped, though, make these key research arenas in ME/CFS. Because long COVID, to all appearances, is probably immune driven, one could see these areas being neglected and one could argue that they are relative to the work being done in ME/CFS, but they are steadily showing up – and, at times, are producing eye-opening results.
The study, “Muscle abnormalities worsen after post-exertional malaise in long COVID“, did something simple but brilliant that we haven’t seen in ME/CFS or FM before. Given the exertion problems, it’s always seemed that something has to be going on in the muscles, and indeed both fibromyalgia and ME/CFS studies (often miserably small) suggest something is, but no one has ever put that idea to the test; i.e. nobody has, to my knowledge, tested how the muscles responded to an exercise challenge until now.
This smallish study (25 long COVID/24 healthy controls) study did. It took muscle biopsies from long-COVID patients (none of whom had been hospitalized) and healthy controls (people who’d recovered from COVID-19) – put them on a bike and exercised them to exhaustion (it doesn’t take long :)) using a CPET protocol – and then took another round of muscle biopsies and compared them.
Led by Rob Wust, an exercise physiologist and mitochondrial researcher, the study was funded by a variety of sources including the Patient-Led Research Collaborative for Long COVID and the Solve M.E.’s 2022 Ramsay Grant Program (!).
The primary aim of that Ramsay Grant was “to unravel the origins of muscle pain, extreme muscle fatigue and post-exertional malaise in patients with long-covid”. I would say the researchers made a good step in that direction. Score a big win for Solve M.E. and their Ramsay Grant program.
Results
Cardiopulmonary Exercise Test (CPET)
First came a standard CPET analysis which assessed how well the participants responded to exercise. The results were pretty typical: the long-COVID patients were clearly inhibited in their ability to produce energy (VO2 max, peak power output). They also exhibited problems moving air in and out of their lungs. The ability to move air in and out is critically important during exercise to remove waste products like CO2 and to supply the muscles with the oxygen that drives our chief source of energy – the aerobic energy production system in our mitochondria.
It was interesting, therefore, to see lower maximal ventilation (a reduction in the ability to pump normal amounts of air at peak exercise) and lower maximal end-tidal pressure of CO2 (PETC02), suggesting that hyperventilation may have been present. This suggests that the long-COVID patients may have been removing too much CO2 from their blood. Too low or too high of anything is damaging, and low CO2 levels can produce many of the symptoms found in long COVID and ME/CFS.
CO2 levels have only recently been assessed in ME/CFS, but the results have been striking. One study found that hypocapnia (low CO2 levels) was far more common in ME/CFS than postural orthostatic tachycardia syndrome (POTS), and other studies have found hypocapnia in long COVID. The biggest exercise study ever in ME/CFS found more problems with “gas exchange” and strange breathing patterns than anything else.
The authors of that study, though, proposed that the real problem probably lay in poor oxygen intake from the muscles and/or problems with blood delivery to them.
The near-infrared spectroscopy readings in the present long-COVID study indicated a reduction in “peripheral O2 extraction” was present; i.e. the muscles of the long-COVID patients weren’t taking up as much oxygen (read energy) as were the muscles of the healthy controls (recovered COVID-19 patients).
All these findings jive with those found in ME/CFS and the reduction in O2 extraction using near-infrared spectroscopy provides a nice validation of Systrom’s invasive exercise findings indicating that problems with oxygen extraction are present.
That was all good, but it was just the prelude to what came next.
Muscle Structure and Function
Next, digging into their biopsies, they assessed muscle structure and functioning. The problems with oxygen (read “energy”) extraction could have been due to reduced levels of blood vessels at the muscles that impaired flows of oxygen-rich blood to the muscles, but that wasn’t the problem, or at least it wasn’t the main problem: capillary density and capillary-to-fiber ratio were similar.
(A trend (p<.08) to reduced low capillary to fiber ratio, and the fact that the ratio was correlated with VO2 max, suggested something might be going on, though.)
Digging deeper into muscle structure, the Dutch researchers found a higher proportion of highly fatigable glycolytic fibers in the long-COVID patients and a lower cross-sectional area of fatigue-resistant type I fibers in females.
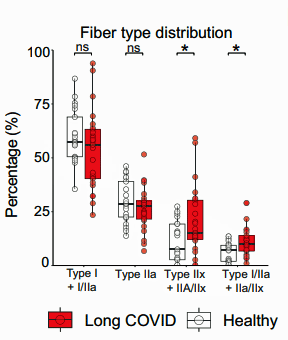
These muscle fibers don’t use oxygen (or the mitochondria) to produce energy; instead, they use a process called glycolysis, which produces energy anaerobically. Not only does glycolysis produce much less energy than aerobic energy production but it leaves behind a substance called lactate, which produces muscle fatigue and pain if not quickly removed.
Systrom, Workwell, Visser, and others have found indications that the aerobic energy production we rely on for the vast majority of our energy is to some degree broken in ME/CFS and long COVID – resulting in a greater dependence on anaerobic energy production or glycolysis. This could help explain why physical exertion is so fatiguing in people with these diseases.
This finding of an increased incidence of glycolytic, or fast-twitch, muscle fibers in the long-COVID patients fits in well with this hypothesis, as does a 2009 ME/CFS study that found increased levels of these “fatigue-prone, energetically expensive” muscle fibers in ME/CFS. Likewise, a 2022 Colorado exercise study suggested increased levels of fast-twitch muscle fibers were present in long COVID.
It was no surprise, given all that, that, ounce for ounce, the muscles of the long-COVID patients weren’t producing as much energy as the recovered COVID-19 patients.
Lower levels of an enzyme, succinate dehydrogenase (SDH), involved in both parts of ATP production in the mitochondria – the citric acid cycle and the electron transport chain – once again made sense given the emphasis on glycolysis (which takes place outside the mitochondria) and the reductions in muscle energy production.
Summing up the section on muscle structure and function, the authors proposed that the lower exercise capacity found in long COVID was in part due to a relative overabundance of “highly fatigable” glycolytic (fast-twitch) muscle fibers and reduced mitochondrial activity, possibly in concert with reduced blood flows to the muscles and hyperventilation during exercise.
Digging Deeper: Mitochondrial Activity and Metabolism
That was all to the good, but with so many illuminating findings staring them in the face, why stop there? They dug deeper and assessed changes in muscle biopsies as well as metabolic signatures in the blood after exercise to see if exercise was taking a hammer to mitochondrial energy production and metabolism.
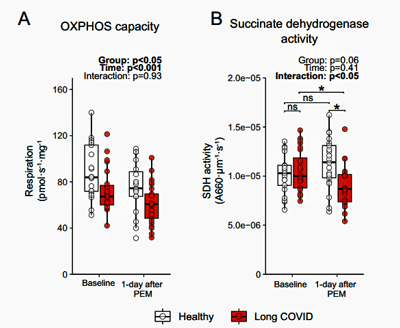
Check out the SDH activity on the right-hand side of the diagram. It increased in the healthy controls but dropped in the long-COVID patients after exercise.
Interestingly, a maximal exercise test one day reduced the ability of both groups of patients to generate energy the next, but the groups separated when it came to succinate dehydrogenase (SDH), with SDH activity increasing in the recovered patients but significantly decreasing in the long-COVID patients. SDH activity had been normal pre-exercise, but the reduced SDH activity after exercise found in the long-COVID patients suggested that exercise had reduced mitochondrial activity and levels.
Muscle metabolism took a big hit. Similar results between the long COVID patients and healthy controls would have resulted in clear circles but almost all the circles associated with the citric acid and glycolytic pathways – were either light or dark blue – indicating lower levels of these metabolites were present.
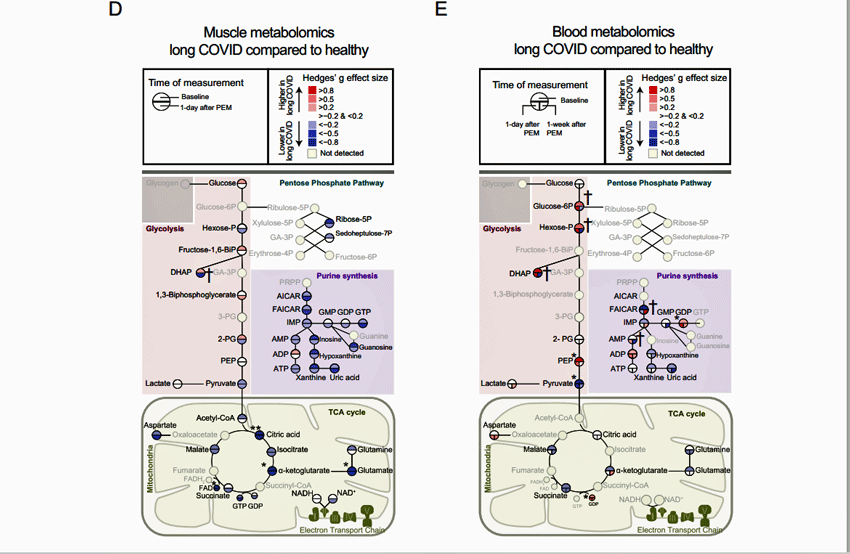
Normal results would have resulted in clear circles…there weren’t many normal results.
The blood metabolomics diagram – which showed a mixture of red (high metabolite levels) and blue (low metabolite levels) – was different. It showed high levels of glycolytic metabolites – suggesting that the glycolytic anaerobic pathway (as suspected) had been activated – but low levels of metabolites associated with the Krebs or citric – suggesting (as suspected) that aerobic energy production had been inhibited by the exertion.
Even at rest, mitochondrial deficiencies turned up with lower levels of several key metabolites (including glutamate, FAD+, alpha-ketoglutarate, and citric acid) associated with the citric acid or Krebs cycle. Note that the goal of the Krebs cycle is to provide FAD+ and NADH to the electron transport chain (which then produces ATP). Alpha-ketoglutarate and citric acid are intermediate metabolites in that cycle. Robert Phair’s Itaconate Hypothesis predicts they will be low in ME/CFS, and so they were in the muscles of long-COVID patients.
The reduced ratio of citric acid (produced in the mitochondria by the Krebs cycle) to lactate (produced by glycolysis outside the mitochondria) in the skeletal muscle indirectly validated the increased levels of glycolytic fast-twitch muscle fiber, indicating that the anaerobic energy production pathway was being emphasized more in the long-COVID patients.
Likewise, lower concentrations of creatine – a key player in energy production, particularly during intense exercise – in the muscles of the long-COVID patients suggested that problems with energy production, particularly during exercise, were present. Some people have used creatine as a post-exertional malaise buster in ME/CFS and creatine has been proposed for use in long COVID as well.
Other findings suggested problems with lipid synthesis, and high levels of oxidative stress may be present.
The idea that microclots could be blocking blood flows to the muscles and other organs has captured a lot of attention. The study did indeed find amyloid proteins (strangely shaped, difficult-to-break-down proteins) in greater concentrations in the skeletal muscle of long-COVID patients but they did not appear to be blocking blood flows.
Resia Pretorius – the originator of the microclot hypothesis – reacted to that finding with alarm stating “”That means the microclots can actually have traveled through the damaged vasculature into the muscle. What is scary, but possibly very significant, is that this might be happening in other tissues as well.”
Nor did they find evidence of low muscle oxygen levels (hypoxia). Still, it was not clear why increased levels of amyloids were found in the long-COVID patients or what effect they might be having.
Digging down into the structure of the muscle fibers, they found that a larger percentage of long-COVID patients displayed necrotic (dead) muscle fibers (36%) after exercise while 80% (!) displayed atrophied muscle fibers after exercise (up from 50% before exercise). It appeared that exercise had induced macrophage (CD68+) and CD3+ T-cells to invade the muscles – something Akiko Iwasaki said is rarely seen in healthy muscles and could indicate an autoimmune response had occurred.
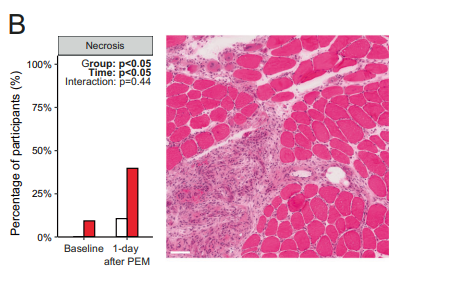
Dead and atrophied muscle fibers in the long-COVID patients after exercise.
Despite the evidence of immune cell infiltration, they failed to find a reason for it. High levels of oxidative stress could have fragmented the mitochondria – drawing the immune cells in, but neither it nor signs of muscle breakdown were found. Nor did the SARS-CoV-2 virus appear to be responsible: similar levels of the SARS-CoV-2 nucleocapsid protein were found in both groups.
Their conclusion that “factors other than viral persistence” are responsible for the muscle damage found clashes with the hypothesis that viral persistence is triggering long COVID. That might not be a bad finding for post-infectious illnesses like ME/CFS, though, which would be faced with figuring out which viruses or pathogens were persisting. In any case, the reason for the exercise-induced muscle fiber atrophy remained a mystery.
Finally, this study used an accelerometer to assess step counts. It found that while the long-COVID patients were rather sedentary (~4,000 steps/day), they weren’t bedbound, and deconditioning could not explain the results; indeed, the muscle atrophy associated with deconditioning was not found.
All in all, this muscle study found evidence of problems at virtually every turn and cemented the idea that intense exercise is harmful. The study findings – which are getting quite a bit of attention – should help doctors and others realize that exercise prescriptions are not the answer. (One wonders what the RECOVER Initiative with their exercise clinical trial is thinking…)
Lead researcher, Rob Wust told the Guardian, “It’s really confirming that there is something inside the body going wrong with the disease. It damages your muscles, it worsens your metabolism, and it can explain why you feel muscle pain and fatigue up to weeks after the exercise,”.David Putrino from Mt. Sinai told NPR “I don’t think the messaging has been strong enough. It is very clear that this is not a typical response to exercise.”
While noting that a gradual exercise prescription can help after the appropriate medical interventions have helped, David Systrom stated “You cannot simply ask these patients to go to the gym and fix the problem.” For his part, David Putrino prescribes what’s called “autonomic rehabilitation“. .
The breadth of the findings – from tissues starved for energy and depleted mitochondria impressed one of he study authors. Braeden Charlton called the energy depletion “very profound“, said “We see this at basically every parameter that we measure.” and stated that “The mitochondria are operating at a severely reduced capacity compared to healthy people,”
The authors noted that post-exertional malaise is specific for long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), and proposed that a similar pathophysiology exists in these two illnesses.
Note that despite the fact that the exercise produced post-exertional malaise in every long-COVID patient, “considerable heterogeneity” showed up in the test results. A similar pattern in ME/CFS suggests that several pathways can produce the same conclusion: reduced energy production and PEM.
THE GIST
- This is the kind of extensive study that we – people with chronic fatigue syndrome (ME/CFS), fibromyalgia (FM), and allied diseases – hoped that long COVID would trigger. The first test of how the muscles of long-COVID patients responded to exercise found problems at every turn.
- Powered, in part, by a Ramsay Grant from the Solve ME/CFS Initiative, the study’s exercise test found evidence of reduced energy production, problems with “ventilation” (moving air in and out of the lungs efficiently), low CO2 levels, and problems with oxygen utilization – all of which have been found in ME/CFS.
- Digging into muscle structure, a higher proportion of highly fatigable glycolytic, or fast-twitch, muscle fibers could help explain why exercise is so difficult in long COVID. (A similar result has been found in ME/CFS.)
- It was no surprise, given all that, that, ounce for ounce, the muscles of the long-COVID patients weren’t producing as much energy as the recovered COVID-19 patients. Lower levels of an enzyme, succinate dehydrogenase (SDH), pointed, once again, to reduced mitochondrial activity in the muscles of the long-COVID patients.
- Exercise only made things worse. Evidence of damaged and dying muscle tissue was found in about a third of long-COVID patients. Muscle and blood metabolomics studies found reductions in metabolites associated with aerobic energy production and an increased emphasis on the dirty and inefficient anaerobic energy production system.
- Other findings suggested problems with lipid synthesis and high levels of oxidative stress may be present. All these findings jive with what we know about ME/CFS and all emphasis the damaging effects of exercise.
- With no evidence of increased coronavirus proteins in the muscles, the authors took an axe to a popular hypothesis when they concluded that “factors other than viral persistence” are responsible for the muscle damage. Finally, the step counts of the participants indicated that the muscle problems found could not be due to deconditioning.
- Two major muscle studies by the Open Medicine Foundation, one of which includes a 2-day cardiopulmonary exercise test (CPET), will tell us even more about this potentially key area of ME/CFS pathophysiology.

The Open Medicine Foundation’s ME/CFS muscle studies are coming at a great time.
Major ME/CFS Muscle Studies Underway Courtesy of the Open Medicine Foundation
The Open Medicine Foundation has two major muscle studies underway under the guidance of David Systrom and Wenzhong Xiao. One consists of a deep, deep dive (genomics, proteomics, metabolomics, phospho-proteomics, ultrastructural analysis, mitobiogenetic markers) into muscle samples from ME/CFS patients.
The next study will go even further than this long-COVID study and take muscle samples before and after a two-day CPET exercise test. Among other things, it will also assess levels of citrate synthase (which Systrom has found depleted in ME/CFS before), gene expression, metabolites and proteins in the muscles, as well as mitochondrial functioning, cytokine, gene expression, metabolites, and proteins in the blood. Given what we just saw with the long-COVID study, this is a timely study indeed.
Conclusion
The study found evidence of muscle damage and energy depletion at virtually every turn.
Studies suggest that energy production is impaired in long COVID but we didn’t know if the muscles themselves were impacted. It appears they are. The first test of how the muscles of long-COVID patients responded to exercise found problems at every turn.
Powered in part by a Ramsay Grant from the Solve ME Initiative, the study’s exercise test found evidence of reduced energy production, problems with “ventilation” moving air in and out of the lungs efficiently, low CO2 levels, and problems with oxygen utilization – all of which have been found in ME/CFS.
Digging into muscle structure a higher proportion of highly fatigable glycolytic or fast-twitch muscle fibers could help explain why exercise is so difficult in long COVID. (A similar result has been found in ME/CFS.)
It was no surprise, given all that, that, ounce for ounce, the muscles of the long-COVID patients weren’t producing as much energy as the recovered COVID-19 patients. Lower levels of an enzyme, succinate dehydrogenase (SDH), pointed, once again, to reduced mitochondrial activity in the muscles of the long-COVID patients.
Exercise only made things worse. Evidence of damaged and dying muscle tissue was found in about a third of long-COVID patients. Muscle and blood metabolomics studies found reductions in metabolites associated with aerobic energy production and an increased emphasis on the dirty and inefficient anaerobic energy production system. Other findings suggested problems with lipid synthesis and high levels of oxidative stress may be present.
With no evidence of increased coronavirus proteins in the muscles, the authors took an axe to a popular hypothesis when they concluded that “factors other than viral persistence” are responsible for the muscle damage.
Finally, the step counts of the participants indicated that the muscle problems found could not be due to deconditioning.
Two major muscle studies by the Open Medicine Foundation, one of which includes a 2-day cardiopulmonary exercise test (CPET), will tell us even more about this potentially key area of ME/CFS pathophysiology.
Last Week of Health Rising’s BIG (little) Donation Drive

Exercise studies are like catnip to us – we always cover them 🙂
Thanks to the hundreds and hundreds of people who have contributed to Health Rising’s donation drive. We are nearing the end.
Nothing excites me more than exercise studies – particularly studies that take their findings to the next level. This study – the first to really test the muscles – did that and its results confirm what virtually everyone with ME/CFS/Long COVID instinctively feels – something has gone very wrong with the muscles. If covering the results of exercise studies appeals to you please support us in a manner that works for you.






The 24 hr pathology test 12 hours after exercise and then a further test 12 hours later. I have results of this testing dated 1998 – it is all about amino acids excess use by the body – not good. Does anyone consider the biochemistry in this cascade of biological dysfunction – P38 MAPK end product of AMPK pathway dysfunction in muscle! Ido not want to give a biochemisty lesson on this subject but I could go further.
Interesting. Many years ago, I had some very specialized blood tests done that reported that my (cellular?) amino acids levels were low. This perplexed me as I have always been a heavy meat eater, which made me assume that my amino acid levels would be higher than average (rather than lower) if not normal. I always considered this unexpected test result to be an abnormality that (if investigated) might lead to a core mechanism in my CFS/Fibro problems.
I guess that fits with one of the other recent articles on Health Rising, in which the researchers observed that we are deriving our energy from amino acids instead of from fats and sugars, which would be a more efficient process.
Elsie.
I wonder if that’s why I’m putting on so much weight?! Fats and sugars are not being utilised?! (Menopause isn’t helping though, either!)
I don’t know, but it’s something I struggle with, too. Between my inactivity, low metabolism, and whatever this food-to-energy conversion issue is, it seems that everything I eat goes directly to fat with no energy production. I have to go on really strict diets in order to lose any weight, and the weight returns easily.
Before I got sick, I was always “that tall skinny girl.” I’m still tall, but skinny is a distant memory.
Me too – I was always skinny, I never thought I’d put on weight like I have and I just seem to keep getting bigger! No point anyone telling me to eat more healthily – I’ve got the ME thing of no longer being able to shop for ingredients or cook, and eating whatever is in the cupboard. Mind you, my mum was slim when she was young and started to put on weight in middle-age so maybe it’s just an aging/genetic thing. The fact I can do less and less physically does seem to bear out this research.
DAVE – This what I have researched during my investigation into my own health problem. Fortunately I eventually found out my problem began years ago when diagnose with a bacteria from birds. What was not made known “the antibiotics do not enter the cells” soI remained in very poor health unable to work after 40 years old. I have written a book Beyond the Birdcage: Inconvenient truths about Myalgic Encephalomyelitis” a copy has been sent to Cort – may not be too interested – but it would bevworth a read. Anyway with low levels of amino acids means your body is using those for energy ATP which is known as anerobic a process that is linked to a glucose and essential fatty acid problem. The scientists are say infections are a cause but nit looking to the testing protocols which are sub standard.⁹
Jennifer, TY for your intriguing response. When I was 12, we were given an Amazon parrot from Trinidad (that was brought here by its owner in the 1950’s before any restrictions, quarantines or health checks). This bird seemed to be sick, as it regurgitated (and lost) its food – sometimes more often than others. I have often wondered if this bird’s “bug” may have instigated or triggered my health challenges. However, when my symptoms first became clinical/severe at age 30, I was also working 2 highly stressful jobs during a stressful time in my life (Type A Personality in high gear), was working in a building with a lot of black mold, and, had come into first contact with the cold sore virus about 6 months before I got sick, so there are many potential triggers for me. Your book interests me, but my eyes are so sensitive that I can no longer read.
Very interesting considering I ran a pheasant farm and had pigeons.
Did anyone see the in the recent news that pigeons carry and can infect humans with a fungus?
Buckey You had a very interesting collection of birds – i would much prefer the turkeys they apparently don’t harbor as many diseases as chickens. All birds carry (Chlamydia psittaci) and disperse it in the air especially when stressed such as in cages or moved from one location to another etc. It is well documented that the disease in humans is called psittacosis; a very old disease with a very troubled history -worldwide pandemic – one important one in the context of ME 1934. For over a century medicine believed the disease was caused by a virus until proclaimed a bacteria in 1966 by L. A. Page. This is my take on testing – if you test positive for Chlamydia pneumonia then I would get treated but the antibiotics do not enter the cell so a supplement is needed to deal with the biofilms that is a protection mechanism produced by the bacteria. I could go further but could write up more from the research in my book 415 references.
It should be noted that all animals/pets carry illnesses that can be harmful to their caregivers, and the dangers that pigeons present are historically GREATLY exaggerated.
(The National Museum of the US Army has a page dedicated to a pigeon saved 194 lives in WWI.)
(And pigeons routinely outperform people, including mathematicians, at learning The Monty Hall Dilemma.)
https://www.thenmusa.org/biographies/cher-ami/#:~:text=Army%20medics%20were%20able%20to,honor%20bravery%20on%20the%20battlefield.
https://www.discovermagazine.com/planet-earth/pigeons-outperform-humans-at-the-monty-hall-dilemma
Your comment, and other’s comments about birds is interesting to me too.
My mum and my nan looked after injured wild birds for a bird sanctuary when I was a child (and I’m sure I got involved!) plus we had budgies, canaries and a parakeet as pets. Later my mum and stepdad kept racing pigeons for many years, of which I helped with timing them in after races (involving handling them inside the loft)
(I’m not aware that racing pigeons carry anything that can transfer to humans, but years of cleaning them out can of course cause ‘pigeon fancier’s lung’)
I wonder if my contact with birds when young – and more recently handling the odd injured wild bird and grounded baby gull, and use of bird feeders (I always wash my hands after) has perhaps impacted on my health and has perhaps contributed to my CFS/ME
(As well as glandular fever, influenza, and stress etc!)
Sandra Your long history of bird connection is to be considered when trying to put the pieces together about your health. It is well documented about bird droppings where the chlamydia can remain for a few months – all birds carry the pathogen. Not to say that birds should be targeted as a problem – it is caged birds and disturbed habitats – birds taken out of their natural environments they are very stressful creatures. I sent a copy to my nonfiction book: “Beyond the Birdcage: Inconvenient Truths about Myalgic Encephalomyelitis” to Cort Hohnson but have had no recognition of him receiving it to date. I believe you would be very interested in the chapters – especially about the birds but also the medical side of the disease if he would be willing to share the research. Fortunately, I was treated by Australian tropical diseases MD along with Lyme patients privately linked to a university. I remain in good health.
Thank you Cort for this full explanarion of this excellent study.
One thing stood out for me was how fast twitch muscles were particularly affected.
Several years ago my 23andme results showed i had fast twitch muscles. In fact i am the only one in my family who are still alive who have fast twitch muscles and the only one with ME.
However my Aunt also had ME and she died in 2022 aged 97 so i wonder are we with fast twitch muscles mire likely to get the illness?
That should be easy to test. People of West African origin are supposed to have more twitch muscles. If they are no more likely to get MECFS, then the premise would be false.
Pam, I too had the fast twitch result with 23&me. My family all fell down laughing.
Most of the recent discussions of red blood cells has been interesting to me with 25 years of lab work to back up the issue, I wish there would be more on this subject.
Also, am I the only one interested in what happened to the CHROME genetic study? They have results of their trial, noting markers.
Excellent study.
I am quietly hopeful that we are within 2 years of some really meaningful progress.
I think that’s a nice timeframe. For me, it was so great to see issues show up in the muscles. We need a bigger study but I would be surprised if the results were much different – the findings make so much sense. I’m so glad the OMF has that 2-day ME/CFS exercise muscle study and honestly, expect similar results.
For now, hopefully, researchers will start trying to figure out what’s causing those immune cells to invade the muscles during or after exercise and keep after the cause of the energy depletion.
A real world reflection of fast twitch (instant energy) vs slow twitch (stamina) muscles?; About a year ago I told my Doc that my energy levels continued to decline. To test my strength he put his elbow on the desk and told me to push against his hand with mine as hard as I could (effectively an arm wrestle). He is huskier than I am, but I beat him quite handily. He was shocked. I explained that “my problem has never been strength” (fast twitch muscles?), “but rather stamina” (slow twitch muscles?), “and, that in spite of no shortage of immediate strength – a 3 block brisk walk would bring me close to collapse (with several days recovering to the pre-exercise baseline functionality).”
“Arm wrestling” the Doctor…Exactly, Dave! I LOVE this example.
Energy/Strength IN an Instant, FOR an Instant!
I can easily open the stubborn lid on a jar of pickles…to the amazement of my family. However, a simple unhurried walk around the neighborhood takes me out (flare up/collapse) for DAYS. I DREAD Grocery Days.
I’ve also noticed that I take a deep breath and hold it while doing things that require exertion. I can’t BREATHE and DO A TASK at the same time.
For years (I’m 55 and was diagnosed with ME/CFS at 19.) I blamed myself for not trying hard enough and for being “weak”. I’m glad to know that Fast Twitch Muscles and Slow Twitch Muscles exist, are being recognized, and will now be part of my vocabulary.
Susan, I love your description; “Energy IN an instant, FOR an instant”.
In a way it makes sense doesn’t it? If the aerobic energy system is not doing well maybe the anaerobic energy system (glycolytic) is getting a bit stronger to compensate (???)
Yes Court. I also think it is a compensatory adaptive response. I am 60 with CFS since 27, and I can beat my twenty year old son in an arm wrestle. But I do not try that often, I am finding that my joints or ligaments do not like that activity.
Cort, I have a question how the energy impairments found in this study relate to the Itaconate shunt hypothesis (you mention in this blog that results in this study also confirm predictions of the itaconate shunt hypothesis):
The Itaconate shunt hypothesis as far as I understood proposes a shift to using amino acids for energy production resulting in ammonia as a “waste product”, and in this study you describe how muscles switch to anaerobic glycolysis resulting in a byproduct called lacate.
My question is, how do these processes (energy production from amino acids, and the anaerobic glycolysis) fit together? – They are not the same, right? Do they both happen in the body, maybe in different tissues? Thank you!
This study is so good – from providing various evidence of impaired energy production/mitochondria to actually visualising, muscle damage post-exercise plus ruling out deconditioning. And your presentation in this blog is excellent, Cort 🙂 I appreciate the connections made – it is encouraging to see different studies coming together researching the same thing. It must be exciting to HAVE more and more findings to crossreference in your blogs!
This study so resonates with my symptoms too, at last! I can’t always get a lid open, and no pull-ups any more, but I can at any time do 20-30 (quick!) push-ups with no problem and no sore muscles.
Having fibromyalgia and life-long, but jab-triggered “MCAS” = “post vac syndrome”, not officially ME/CFS.
My main remaining symptom from both conditions is what I call a “fast exhaustibility” type of fatigue, with a severe overall muscle Ache. Which however feels different to “sore muscles”, so I don’t think lactate, as suggested by the study, is the reason.
It was the recommendation (here too) to try LDN for all of these conditions – fibro, ME/CFS, Long Covid, post vac and MCAS that finally got me to start on it. Starting very low (0.07mg) kept side effects to a minimum and immediate and at last stable increase of energy from basically “10%” to “20%”. now up to 2.5mg and up to “30%” – but only when I take it. It has also improved my sleep even more (but have to take it in the mornings).
A tub of creatine has been sitting on my desk for a year or two now, untouched. But the study and Cort, your comment, has now let me look at the studies for it, and I’m beginning to think it makes sense to at least try it. My tub is the monohydrate form, but this article https://poweringo.com/monohydrate-or-malate-which-type-of-creatine-to-choose/ suggests the malate form may be better for those of us with this quick exhaustibility. And this study by Longobardi et al. 2023 seems to quench the fear of kidney problems: https://pubmed.ncbi.nlm.nih.gov/36986197/
A stupid question that popped up in my head. When looking on the population that gets long covid they have often been very active, even in sports, do that population have genetically different coctail of muscle types?
And if how does that correlate to the study’s results? Are sprinters more or less likely … and to what?
As one often talk about people with clinical burnout often caracterized as type A-personality?
Succeptability?
Yes, Kajas! This makes a lot of sense to me.
I’m starting to think that those of us who develop ME/CFS or Long COVID were almost DESTINED to develop this condition because of Muscle Types we were BORN WITH and have been living with all our lives and that the Virus simply activated or woke up something that has been in us all along.
I’m 55 and was diagnosed with ME/CFS as a teenager. From the very start, I’ve been the typical Type-A, Over Achieving personality. I’ve always been the “Sprinter” who could accomplish a lot in a short period of time…at least until the Crash hit. I wonder if DECADES of pushing yourself to the Limit causes the seemingly Irreparable Damage we feel?
In response to Kajsa and Susan; Interesting! Personally, I can relate to the suspicion that CFS/Fibro/ME (and Long Covid) sufferers may have a genetic predisposition to these diseases – perhaps relating to or including a higher proportion of fast twitch vs slow twitch muscles. I also wonder if those with a higher proportion of fast twitch muscles might also (somehow or thereby) have a greater tendency towards anaerobic versus (more efficient) aerobic energy production? Might these two characteristics be related? Personally, I first noticed that I had greater strength but lower energy stamina than most of my peers even in childhood (which shaped my choice of sports, and the way that I played them). Then at age 30 (under multiple stresses), something major in my metabolism, energy and immune response changed (very suddenly) – which evolved (over a few months) into the classic symptoms of (significant) CFS and Fibro. (Could this change have included a triggered shift from (predominantly) aerobic to anaerobic energy production perchance?)
It is still unclear why the muscle tissue of long Covid patients differs from that of healthy people. more research into PEM is needed. It is not yet established whether the same findings also play a role in PEM in ME/CFS and other diseases. Still, I think that a double exercise test can objectify ME/CFS and Long Covid more when making a diagnosis. This in combination with detecting reduced blood flow to the brain. Rob Wust also conducts research into ME/CFS and has received funding for this.
https://projecten.zonmw.nl/nl/project/adaptaties-de-skeletspier-en-bloed-tijdens-post-exertionele-malaise-patienten-met-mecvs
I agree – this “why the muscle tissue of long Covid patients differs from that of healthy people” is the question. Mitochondrial damage would have been a great reason but they didn’t find signs of that but I imagine this study is not the final answer. There’s also the possibility of an autoimmmune response. The pathogen persistence idea took a hit, though.
Glad to hear that Wust is doing ME/CFS work! Here’s what the study will do.
They have to persist to not acknowledge the latent phase of disease because to do so destroys the 1994 CFS definition that states Lyme disease is cured by antibiotics. I know for a fact that antibiotics do not cure Chlamydia because they do not enter the cells. I would need to reference that statement – K. F. Meyer, The Natural History of Plague and Psittacosis, American Journal of Public Health, p.573.
Good, you mentioned the MuscleME-study already. My friend Peter was so happy to be a study object that he made a t-shirt 😉 https://twitter.com/ValeBodi/status/1710291094808809838
Good Summary Cort,
Linking various previous Studies to this one makes it more comprehensive.
🙂
I too really enjoy the connections you make, as previously with comparing fatty acid metabolism coming up in different studies.
I think there is an alternative explanation for this. Sick people have difficulty generating energy (ventilation problem?). long-COVID patients are sick, and therefore they have difficulty generating energy for exercise. Exercise made the problem worse obviously, because they become much sicker afterwards. As for the muscle damage, the subjects were probably deconditioned badly. And deconditioning will cause substantially more damage after a maximal exercise. I would be more convinced if they used other sick people, say flu patients, as control, not healthy or recovered patients in the next study.
That said, this is yet another in the long series of findings that MECFS is a physiological disease. Anybody who still thinks MECFS, or long-COVID, is all in the heads should have their heads checked.
Wust was clear that this was not the result of deconditioning. For one the long COVID patients averaged about 4000 steps a day – which is about 20% below the mean for the average American but just about enough to increase longevity according to some studies. That would suggest that their muscles probably wouldn’t respond differently to an exercise challenge.
Systrom has also reported that his exercise findings are opposite to those seen in deconditioning.
I’ll take his words for it. But 4000 steps/day is hardly enough a conditioning for a maximal exercise. I remember cramping all over just walking to the ticket booth, and then sore for several days after getting back on the bunny slope in 2019. That was when I was taking 10,000 steps during the summer while traveling. I’m sure a sports physiologist knows better than I do, but cardio conditioning is not same as muscle conditioning. Someone who can run marathon won’t necessarily last 30 seconds in a wrestling match and then ends up with muscular soreness for days.
Do you have the link to Systrom’s finding, Cort?
TK, I am a long COVID patient and I had the two-day CPET in 2022, after being sick for two years. I had zero problem with reaching maximum effort on the exercise bike on day one, which really shows that deconditioning is not the issue. It was my measurements on day two of the test that really showed my body tanking.
That’s what I’ve been saying all along: MECFS patients don’t have problem generating energy. Only problem is that they keel over the next day for a week or two after doing 10-100W in 10 min. Being able to generate 100W doesn’t mean that your muscles are in shape though. If you’ve been taking 3-4000 slow steps with frequent rests and not much else, you are going to be sore after doing 100W, though I’m not sure 1 min would be enough.
This kind of skepticism can be easily addressed by using the similarly deconditioned/sick people as control. Using healthy control and then assuring that deconditioning is not a factor in the muscle abnormality just don’t fly for me.
https://pubmed.ncbi.nlm.nih.gov/33947430/
Deconditioning does not explain orthostatic intolerance in ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome)
“Deconditioned people, ironically, exhibit an opposite finding (increased as opposed to decreased filling pressures) to that found in this study.”
https://www.healthrising.org/blog/2016/07/04/exercise-intolerance-fibromyalgia-chronic-fatigue-pots-explained/
The authors reported that the study “definitively eliminates (the) possibility” that deconditioning is causing the exercise abnormalities “because the hallmark of deconditioning is low peak exercise cardiac output” rather than the increased output they found.
Plus, instead of the high heart filling pressures seen in deconditioning, they found the opposite – low filling pressures. Plus, they noted that deconditioning doesn’t have any effect on oxygen extraction, which was low in the low-flow patients.
https://www.healthrising.org/blog/2021/05/06/small-nerve-fiber-snf-energy-chronic-fatigue-syndrome/
I am definitely not decontitioned because I can do 10000 steps some days and average 8000 a day unless I have a virus which is quite frequent in winter.
However this only happens because I have many rest periods throughout the day plus I take some supplements that help the mitochondria work plus I use an oxygen concentrator at least 3 times a day and I am always horizontal a good part of the day.
Despite this I have NEVER been able to walk for longer than 20 minutes non stop in over 24 years and I have the fast twitch muscles. Any activity that requires on going exertion very quickly completely exhausts me and leaves my legs leaden.
So I firmly believe the deconditioned theory is complete rubbish when related to ME and probably LC too.
Good example, Pam. I very much agree.
Really all I can say is I wish someone would recognize this for fibromyalgia “While noting that a gradual exercise prescription can help after the appropriate medical interventions have helped, David Systrom stated “You cannot simply ask these patients to go to the gym and fix the problem.” For his part, David Putrino prescribes what’s called “autonomic rehabilitation“. .” And that they would include fibromyalgia patients in these studies to look at similarities. We have major muscle, exercise, and fatigue issues.
Lets not forget that EBV reactivation has been found with exercise…
So it may be that the reactivation if triggering the immune system, etc.
The question what may cause the extensive muscular damage is intriguing.
This could be a clue: the central defect in ME/CFS is the lack of a normal stress response. ((https://www.kinder-verstehen.de/wp-content/uploads/Literature-on-the-stress-response-in-ME.pdf )) The physiologic stress response prepares the body for the untoward effects of stress like inflammatory stimulation, oxidative stress etc.
It is not surprising then that Maureen Hanson´s team has shown that inflammatory signalling is increased after exercise in ME/CFS patients compared with controls ((https://isevjournals.onlinelibrary.wiley.com/doi/epdf/10.1002/jev2.12403 )).
In their analysis of extracellular vehicles they even give some mechanistic insight how muscle damage may occur through exercise in ME/CFS: healthy people react to exercise with a rise in several proteins which are thought to be protective as they can induce repair processes in muscle cells (like actin cytoskeletal proteins). This protective surge is lacking in ME/CFS patients.
This could be the reason for the very intense muscular damage seen in many patients with ME/CFS. It would be a good idea to try to correlate the level of actin cytoskeletal proteins with histological findings.
Maybe Rob Wust and Maureen Hanson could collaborate here.
Seems like a very sensible study. Thank you Dr Wust, colleagues and funding organisations!
And of course a big thank you to the participants as well!
…..and why isn’t any research being done up inside the nostrils and further up the airway where viruses get their foothold. My nostrils are a mess
The nose is actually getting some research and it’s prett darn interesting research at that. Check these out 🙂
https://www.healthrising.org/blog/2019/10/14/phantom-nasal-congestion-chronic-fatigue-fibromyalgia/
https://www.healthrising.org/blog/2020/07/11/mucosal-genes-chronic-fatigue-syndrome/
Has any research been done in the blood.
I’ve recently been in touch with a wildlife biologist, that after digging into my health, tells me I have severe blood contamination that is complex but resolvable.
I knew a man several years ago that had me/cfs that cured his with herbs
Thanks for this, Cort.
Also not sure if mentioned but there is a US. Senate Hearing on Long COVID next week, that will be live-streamed
The HELP Committee in the US Senate will be holding a “Addressing Long COVID: Advancing Research and Improving Patient Care” hearing next Thursday, Jan. 18th.
https://www.help.senate.gov/hearing…advancing-research-and-improving-patient-care
**Date:** Thursday, January 18th, 2024
**Time:** 10:00am
**Location:** SD-430
Trying link again:
https://www.help.senate.gov/hearings/addressing-long-covid-advancing-research-and-improving-patient-care
If researches want to do this study with ME?CFS patients, I would volunteer. I had a difficult time getting through this article because of the cognitive effects of CFS, and will need to read it more than once to absorb more of the info. If I’m understanding this correctly, there’s very useful information from this study that could point the way to actual treatments someday. I also find it a bit depressing that the loss of muscle strength I am experiencing may never be regained, and may not just be the result of my lack of exercise caused by CFS, PEM, fatigue, and pain. sigh
Cort, I know that people are born with higher or lower proportions of fast-twitch muscle. Was the research suggesting that the illness increased the proportion of fast-twitch muscle, or that those who had a higher proportion were more at risk of these problems?
(Sorry if this was obvious and I missed it.)
Cort – thank you very much! For remaining questions I had a look at the study, and saw I’d misread you a few times. However you wrote “(36%!) displayed atrophied and dead muscle fibers after exercise.” But in the study (fig. 5, A and B) it seems to me, 36% is for necrosis alone (B), up from about 10% baseline, whilst atrophy a day after exercise (A) is up to around 80%(!), from around 50% baseline (which seems high already). Not sure if everyone with atrophy had necrosis, but I’d assume so.
Thanks JayCS 🙂 I will fix it.
Glad to help! And they had 21 controls, not 24… ¯\_(ツ)_/¯
(4 healthy controls withdrew, so it was aimed at 25:25)
Pity that they don’t directly specify which subgroups of Long Covid the 25 belonged to, just “severe long COVID”, healthy previous to CoV, and not hospitalized cos of it.
Well-known German virologist Streeck in a talk (2023-10-05) said “post / Long Covid” is actually a potpourri of
1) having had Covid severe enough to keep more permanent indirect effects, e.g. from intubation,
2) virus persistence [like we know from EBV],
3) autoimmune reactions caused by the spike protein, “probably similar to the post vac syndrome” [but later in that talk he put PVS in with Long CoV],
4) no somatic cause found, so psychosomatic. [Errhm…]
But it does seem clear that post vac wasn’t included in this Appelman/Wüst study, nor #1 or #4(?). A nd that they deliberately didn’t try to distinguish between #2 and #3 beforehand, because those are still hypothetical and they are studying exactly that(?)
Streek is controversial I think (I don’t remember for sure, but wasn’t he in the “Covid’s not that bad, take it easy with the Corona protective measures” crowd, and his pandemic predictions were usually more optimistic than true?). Lots of publicity though.
True (and I don’t side with him), but distinguishing types/groups of Long Covid like he and others do would still be helpful, like: did the 25 patients selected have more respiratory issues and more ME/CFS-issues than other Long Covid patients? And was post vac excluded?
Zhang et al. 2023 had a 4-fold grouping by symptom types https://covid19.nih.gov/news-and-stories/researchers-identify-four-long-covid-categories /
https://doi.org/10.1038/s41591-022-02116-3,
and Canas et al. 3-fold, similarly (neurological, respiratory and systemic/inflammatory + abdominal) https://www.medicalnewstoday.com/articles/what-are-the-3-main-types-of-long-covid and https://www.medrxiv.org/content/10.1101/2022.07.28.22278159v1.full
Agree on importance of subgroups and let’s hope for virologist’s learning curve to clearly label ME/CFS type as one 🙂
Why would this only be applicable to the muscles and not also to the CNS/Brain? The same fatigue occurs in the latter with mental exertion as with physical in the muscles. Thats why I think its a mitochondrial issue and not something something more exclusive to muscle physiology. I do understand that its easier to test this in the muscles however.
Can someone explain whether ketosis/ketones could assist the energy problem in me/cfs and LC? My understanding is that something is largely keeping the glucose only in the anaerobic phase and not into the Kreb cycle (2 ATP vs 32). Could fatty oxidation re ketones help or is that too far into the Krebs cycle?
Yes! This makes so much sense to me! For years I have tried to describe the fatigue I feel as somehow involving some defect in my Krebs cycle. I think this also helps to explain the low levels of cortisol and amino acids – cortisol is intimately involved in converting amino acids to glucose (gluconeogenesis). And I’ve also found that the NADH supplement I’m taking seems to help with the fatigue.
Our support group has had the privilege of Professor Sonya Marshall-Gradisnik’s presence for over the past 10 years. She heads a research program at Griffith University on the Gold Coast in Australia. (The NCNED – National Centre for Neuroimmunology and Emerging Diseases)
She and her team have currently proven that the receptors on immune cells that draw calcium into the cells are impaired, for 100% of people with ME/CFS, which explains a great deal about ME/CFS.
Dr. Sarah Myhill and Dr. Jacob Teitelbaum have both been treating people with ME/CFS for over 30 years and both believe that the primary underlying cause of ME/CFS is impaired mitochondrial function.
If you are not already familiar with these issues then I would encourage you to explore them.
Your sincerely Sue Bernardo
Great treatise of “how” but never suggesting “why” such as a specific pathogen (that was perhaps reactivated by SARS-CoV-2 or by mRNA inoculation injury).
Since the human body naturally seeks homeostasis, then there must be a pathogen or malnutrition that is interrupting the healing process. Regardless, it is oxidative stress of the cells that precludes homeostasis and good health.
Solution, assist the immune system in recognizing and killing the offending virus with ultraviolet blood irradiation and ozone treatment, and then followed with both IV-C and IV-magnesium to return cells to health by eliminating their intracellular oxidative stress.