

THE GIST
- Major Takeaway – a new hypothesis emerges suggesting that an overactivated immune system may be stealing resources from the rest of the body. A readily available assay is available to test that hypothesis and provide a way to easily test possible drugs
- Mark Davis – a Stanford immunologist – and Ron Davis scored a rare large NIH grant to use some cutting-edge technology to study T-cells and HLA genes in ME/CFS back in 2018. Nothing has been heard from Mark Davis since then until now.
- It was assumed that the grant didn’t work, and in fact, it mostly didn’t. It was saved, though, by an enterprising graduate student named Vishnu Shankar, who excited by what he’d learned about the energy depletion found ME/CFS, made it his mission to explore that aspect of T and B-cells in ME/CFS.
- His goal was to find a molecular marker for fatigue – perhaps the most nebulous symptom in the book – but not just any fatigue – the fatigue that results from an energy deficit: the energy depletion-associated fatigue in ME/CFS.
- He focused on oxidative stress; i.e. unbalanced molecules called free radicals or reactive oxygen species which in an attempt to balance themselves can rip electrons out of cells and damage them. Oxidative stress is a natural outcome of energy production and, indeed, most of it comes from the mitochondrial engines that power our cells.
- Because high levels of oxidative stress can damage immune cells, Shankar and Davis first looked for evidence that high levels of oxidative stress were present in the T and B cells of ME/CFS patients – and found it – but not in men, only in women.
- Moving forward to assess the system designed to keep antioxidant levels in check – the antioxidant system – they found evidence of dysregulation in both men and women with ME/CFS. The unusually high levels of antioxidants in both sexes’ immune cells suggested they were both battling to contain the high levels of oxidative stress present.
- While the cause of oxidative stress was not identified it was likely due to mitochondrial problems. This is because immune cells have to dramatically ramp up their engines to deal with pathogens – thus putting a lot of strain on their mitochondria. In fact, the immune system is one of the most energy intense systems in our bodies. Because of this, if mitochondrial problems are going to show up, the immune system is a good place to look for them.
- Taking a deeper look they found that higher levels of oxidative stress in the female ME/CFS patient’s T-cells resulted in increased T-cell proliferation – not necessarily a good thing. That’s because producing more T-cells requires more energy. That puts a greater strain on ME/CFS patients’ possibly already damaged mitochondria. The oxidative stress produced by the damaged mitochondria could further damage the mitochondria – and that possibility formed the basis for the authors’ model of what may be happening in ME/CFS.
- Davis proposed that an infection triggered increased immune cell proliferation. Over time that proliferation put too much stress on the mitochondria – causing them to break down and release high levels of free radicals/reactive oxygen species. Those high levels then damaged the mitochondria – causing even more oxidative stress – and throwing people with ME/CFS into a vicious cycle.
- Adding antioxidants like NAC, metformin, and liprostatin-1 provided proof for their idea that oxidative stress may causing high levels of immune cell proliferation in ME/CFS. While studies do suggest that metformin may help with ME/CFS/Long COVID, Davis felt these options were the beginning of the road – not the end. He noted that the cell cultures he was using could also easily be used to assess many other drugs.
- This is not the first study to find energy problems in our immune cells. At least three other ME/CFS studies have found that our immune cells are having problems producing energy.
- Davis proposed that by using up all this energy, the immune system is operating as an “energy sink” thats diverting energy from other parts of the body. This is, in effect, what happens when you have an infection – everything goes into fighting off the infection. That would appear to be a very nice solution, if correct! If the immune system is the driver of ME/CFS we have only to deal with it – not the myriad other systems in the body.
The field feels like it’s beginning to cohere around pathways involving energy metabolism, lipid metabolism, and gut and immune issues. Parts of the brain (brainstem, motor cortex, limbic system, prefrontal cortex) have shown up multiple times. While progress is inevitably slow in such a complicated and poorly funded disease, ME/CFS researchers slowly but surely seem to be closing in on some key features.
One would like to think that progress in any field would be rewarded by more funding but not at the inexplicable NIH – not yet. While NIH ME/CFS funding rose from 15 million in 2017 to 17 million in 2021, it has since then fallen to $13 million and is projected to stay there. The 3 small NIH-funded ME/CFS research centers were (somehow) supposed to help increase funding, but ME/CFS research at the NIH is in decline.
This conference amply demonstrates why the NIH is such a maddening institution. The scintillating results from some of the NIH-funded studies simply underscore how much faster we could move on this disease if the NIH chose to throw a bone the ME/CFS community’s way. Former NIH Director Francis Collins promised 8 years ago that the NIH was finally going to become “serious” about ME/CFS. It still clearly is not. Whatever has been done is not working.
Onto better things. In this blog, an NIH-funded study first goes bust – and then turns up roses.
Highlights from the December 2023 Conference on “Advancing ME/CFS Research: Identifying Targets for Intervention and Learning from Long COVID”
Mark Davis
How nice to see Mark Davis again! Some may remember that Mark Davis – a Stanford immunologist – and Ron Davis scored a rare large NIH grant to use some cutting-edge technology to study T-cells and HLA genes in ME/CFS back in 2018. Nothing has been heard from Mark Davis since then.

Vishnu Shankar, an enterprising graduate student, turned a struggling NIH grant into a win.
I assumed that the study didn’t work out, but here Mark Davis is presenting at the NIH conference – not on T-cells or HLA genes – but on oxidative stress! Indeed, Mark Davis did not produce any results from his study – but did talk about the results of a PhD student of his, Vishnu Shankar, who came to ME/CFS after reading “The Puzzle Solver” a book by Tracie White on the Ron Davis and his search to solve ME/CFS.
Struck by the idea of an energy depletion disease, Shankar got to work. Davis described an enthusiastic and curious researcher who, he said, went to every department possible at Stanford to learn about the mitochondria.
The goal was to find a molecular marker for fatigue – perhaps the most nebulous symptom in the book – but not just any fatigue – the fatigue that results from an energy deficit: energy depletion-associated fatigue.
That’s about as close to the holy grail in ME/CFS needs as you can get. A biomarker for energy-depleted fatigue states would: a) tell us more about how the fatigue is produced, but more importantly perhaps; b) would give pharmaceutical solid ground to test out their drugs in ME/CFS.
I recently talked with a pharmaceutical executive about why drug companies are so reluctant to take on ME/CFS – and the lack of a biological marker they can use to test their drug’s effectiveness is right there at the top of their list. (Blog coming up.)
The Oxidative Stress Gambit
High levels of oxidative stress can tear holes in cells damaging them.
Davis noted that for all the focus on the energy usage of the brain, the immune system uses every bit as much energy as it – and during an infection – it uses more – a key distinction to keep in mind. So, into the immune system, they went.
Then it was onto “reactive oxygen species” (which I grew up learning to call “free radicals”). These unbalanced oxygen-based molecules (hydroperoxide (O2H), superoxide (O2-), hydroxyl radical (OH), peroxynitrite (ONOO-)) have an unpaired electron in their outer shell. Seeking to achieve balance, these oxygen-based molecules rip holes in the proteins, lipids, and DNA in our cells.
Far from being something foreign or even unwelcome, they are an inevitable byproduct of aerobic energy production (note their oxygen base), and we use them to good effect in our immune system to kill pathogens and infected cells. In the same way that a campfire that provides welcome heat can turn into a raging forest fire, too many ROS can, by damaging lipids, proteins, etc., in our cells, produce more ROS, which then feeds the flames.
The goal is to not get rid of ROS – the goal is to keep them in check and manage them properly. Many studies have shown that people with ME/CFS are not doing that. High levels of oxidative stress may be the most consistent finding in all of ME/CFS and Martin Pall formed his ONOO hypothesis for ME/CFS from them.
Given the potential connection between high levels of oxidative stress and its possible effects – reduced mitochondrial activity/energy production, an impaired immune response / cellular membrane damage (lipids) / altered cellular signaling – all of which have been seen in ME/CFS – the idea that high levels of oxidative stress are causing problems in ME/CFS makes perfect sense.
Oxidative stress, though, has always seemed like a rather nebulous solution to this disease. Lots of diseases (cancer, cardiovascular diseases, neurological diseases, kidney diseases, respiratory diseases, and rheumatological diseases) also feature increased levels of oxidative stress. If you have a chronic disease, it’s not unlikely that you will also have increased oxidative stress.
The question is not whether oxidative stress is present in ME/CFS, but what might make it special. What might make it a core part of this disease instead of a kind of secondary byproduct? Problems with the mitochondria could. Davis explained that 90% of the reactive oxygen species are produced in our mitochondria and the mitochondria, of course, and energy production is an active focus of study in ME/CFS.
The Male/Female Dichotomy Shows Up Again
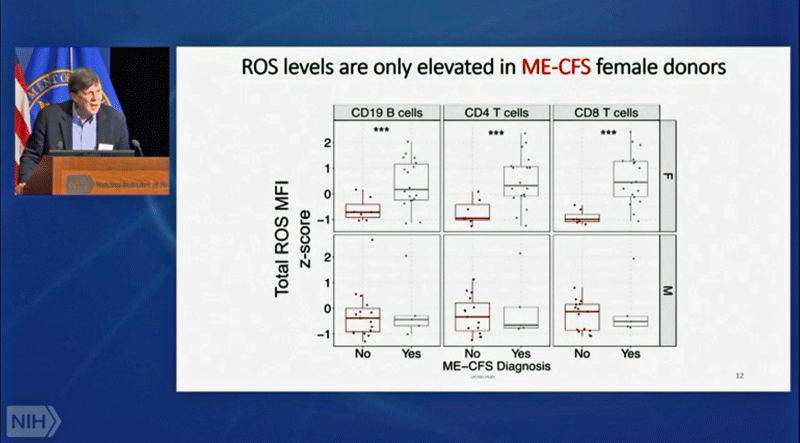
Once again, we see dramatic differences – this time in oxidative stress levels in female immune cells – once ME/CFS patients are separated according to gender. (Females on the top – males on the bottom.)
Back to the T-cells we went. Most immune cells stay in a quiescent state until they encounter a pathogen and then rev up their engines to pump out. Once that happens, they have to rev up their engines and start pumping out cytokines, more immune cells, more ROS, etc.
Looking at T and B cells, Davis and Shankar quickly bumped into the male/female dichotomy that is rapidly becoming a key feature in ME/CFS and long COVID. While male immune cells of people with ME/CFS or long COVID did not show high levels of ROS, females did. (Don’t worry, men – your turn is coming.)
Our cells, of course, evolved to take care of the dangerous compounds (ROS) produced during energy production. Since ROS shouldn’t be a problem in a healthy cell, the researchers looked at the enzyme tasked with keeping them under control – glutathione peroxidase – and found very high levels in both the males and females of the ME/CFS patients and long-COVID patients compared to the healthy controls.
The antioxidant systems of both men and women with ME/CFS, then, were going full bore trying to keep the reactive oxygen species (ROS) in check. In men, that seemed to be working – the levels of ROS levels in their immune cells were normal, but not in women – their immune cells were loaded with reactive oxygen species (ROS). Something, then, was tweaking the antioxidant systems of both the men and women – and that something was probably damaged mitochondria.
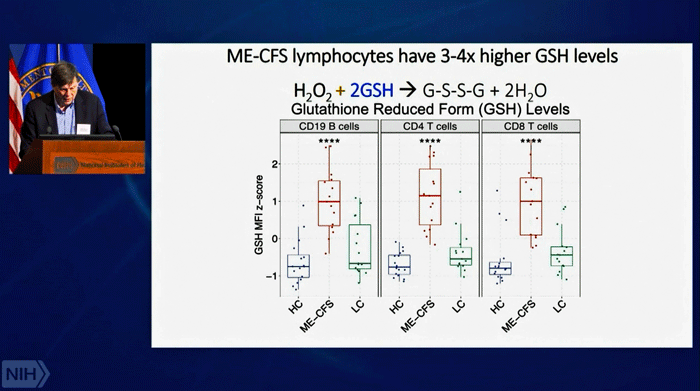
Dramatically high rates of antioxidant production in the immune cells of BOTH sexes, though, suggested that both sexes had ramped up their antioxidant defenses – possibly in response to a mitochondrial problem.
When they looked at ROS production and T-cell activation, something interesting became clear. The higher ROS levels in the female ME/CFS patients resulted in increased T-cell proliferation – not necessarily a good thing.
Producing more T-cells requires more energy – putting a greater strain on ME/CFS patients’ possibly already damaged mitochondria – producing even more reactive oxygen species (ROS) – and potentially more damage to the mitochondria.
(Could this increased T-cell proliferation combined with reduced energy production be another version of the “wired but tired” processes we’ve seen where systems that are always engaged and never rest poop out quickly when called to respond?)
Treatments?
The big question, of course, is whether there’s a way to calm these immune cells down. Note that Davis isn’t talking about curing the problem – we haven’t gotten to the root of the problem (the presumed mitochondrial dysfunction) – but what about bringing down the reactive oxygen species levels in the meantime by calming our immune cells?
They found that adding ROS inhibitors like NAC, metformin, and liprostatin-1 did reduce T-cell proliferation. Metformin, of course, was found to help some people with long COVID, and NAC was found to help some people with ME/CFS, but Davis felt these options were the beginning of the road – not the end. He noted that the cell cultures he was using could also easily be used to assess many other drugs.
In a major paper on ME/CFS, Bindu Paul, Martian Lemle, Tony Komaroff, and Solomon Snyder laid out how problems with oxidative stress could be causing ME/CFS and proposed that much more effective antioxidants are possible as well.
The Energy Sink Hypothesis
If the immune system is using up all this energy, it could produce an “energy sink” that’s diverting energy from other parts of the brain. This is, in effect, what happens when you have an infection – everything goes into fighting off the infection. Given that, Davis speculated that we will not see high ROS levels or mitochondrial problems in brain tissue. We should know the answer to that in the not-too-distant future as RECOVER analyzes lots of brain and other tissues in their long-COVID autopsy.
The Beginnings…
Speculating why some people come down with ME/CFS after an infection, Davis proposed that mutations in perhaps thousands of genes could result in a prolonged level of T and B cell activation. The high energy requirements to meet those needs – and the high levels of reactive oxygen species (ROS) that would inevitably result – could ultimately damage the mitochondria, resulting in an inability to produce normal amounts of energy.
It could also be the inability ME/CFS patients’ immune systems to clear the infection could result in long periods of immune activation, and damage to the mitochondria and energy production systems.
A Marker of Energy Depletion-Induced Fatigue?
Did we get a molecular marker of energy depletion-induced fatigue? It’s possible. Davis said the assay that assessed ROS, glutathione peroxidase, and T-cell proliferation is not a difficult one.
Davis reported that he’d worked on ME/CFS for 6 or 7 years (that big NIH grant) without success until Vishnu, with his single-mindedness and passion, brought this new approach to the fore. How gratifying it is to merge two major facets of ME/CFS – immune dysfunction and energy production – together.
Thanks to Vishnu then, and thanks to Mark Davis for supporting him, and to the Khosla Foundation and NIH for supporting them. Davis also acknowledged Ron Davis, Mike Snyder, and Hector Bonilla.
One would think these findings deserve a nice, juicy NIH grant.

Could the immune system be sucking energy from other parts of the body?
Immunometabolic Findings in ME/CFS
This isn’t the only study to plug together immune functioning and energy production. Alerted by increased levels of a stress factor on B-cells, Geraldine Cambridge in London has been digging into B-cells and energy production for quite some time. Taking a different approach, she and Chris Armstrong recently found that an ongoing “stress response” (immune activation?) was associated with reduced mitochondrial mass, and dysregulated energy metabolism in ME/CFS.
Likewise, several studies, including one from Jessica Maya at Cornell, suggest that T-cell exhaustion is present. Maya’s study was funded by Vinod Khosla (again!), the Alfred P. Sloan Foundation, and the NIH, and a large NIH-funded T-cell exhaustion study is underway. Given the Davis/Shankar findings, it was intriguing to find that an antioxidant (i.e., a reactive oxygen species inhibitor) helped reduce markers of T-cell exhaustion in a small ME/CFS study that was funded by patients (Ramsey/Solve M.E. Award, Open Medicine Foundation, and the Invest in ME charity)
Slowly but hopefully, surely researchers are finding that the energy depletion found in ME/CFS patients is also found in their immune cells. Could the immune system be an energy sink, sucking the energy from all the other systems in ME/CFS? Time will tell, but that could be a very nice answer. Being able to concentrate on just one system in the body would be oh-so-helpful in this complex disease.






This sounds very likely from my experience because Sometimes I am prescribed steroids for another auto-immune complaint and that makes me have energy to live a normal life.
I have had immunoglobulin (for another issue) and the boost to my immune system gave me surplus energy to power the rest of me.
This sounds like we are finally getting somewhere! But what is causing the immune system exhaustion? In many cases it is an ongoing infection such as Epstein Barr virus. Can researchers try CAR-T therapy to address the CAUSE of the immune exhaustion? Sign me up! I’ll be happy to be the lab rat.
🙂 One kind of nice possible cause is a virus that ends up whacking the mitochondria. I believe Bindu Paul suggested that in her redox (oxidative stress) ME/CFS paper and Bhupesh Prusty has been digging into the angle. A 2015 model of mitochondrial dysfunction suggested these factors:
https://www.healthrising.org/blog/2015/05/26/mitochondrial-depletion-could-underly-energy-problems-in-chronic-fatigue-syndrome/
You could be right Cort. 🙂
An initial viral trigger followed by a vicious circle of oxidative stress affecting the mitochondria. And once the “energy sink” is causing immune exhaustion, it then weakens the immune system to defend itself against other viruses/infections and viral reactivations.
Hi Wayneo, the most advanced and supported hyothesis to the question what is the immune system fighting against is that ME/CFS is HHV-6b brain inflammation. It is supposed that there is an initial problem with the immune system after an infection (a specific type of T-cells is at the center of that research) which then allows HHV-6b to wake up and do damage to the brain, the vessels, the mitochondria and the immune system.
Bupesh Prusty together with Mark Williams and Maria Ariza and others were able to come forward with this theory and have beautiful research results. The most interesting ones are a study that shows how enzymes that get produced early on in the HVV-6b reproduction process hamper and even may destroy proper mitochondrial functioning. Link: https://pubmed.ncbi.nlm.nih.gov/32327453/
Then a study by Prof. Jacqueline Cliff of Brunel University in London who was able to show with her team that there was indeed HHV-6b to be found not in the blood(!) but in the saliva of ME/CFS patients and that the virus load correlated to symptom severity (it makes a lot of sense that it would show up rather in the saliva than in the blood given that as far as I know herpes transmission route is smear infection).
However, they could not decide whether HHV-6b was the driving pathomechanism of ME/CFS or a consequence of a yet unknown more foundational problem.
After that came again a study by Bupesh Prusty and colleagues where they were able to detect the typical m-RNA mix that is produced in HHV-6b reproduction in brain tissue of patients who have died and had ME/CFS: https://pubmed.ncbi.nlm.nih.gov/36589231/
Currently I am awaiting eagerly the results of a new and pretty big and carefully designed study by Prof. Cliff and her colleagues: https://www.brunel.ac.uk/research/projects/reactivation-of-herpesviruses-in-chronic-fatigue-syndrome
Here they are now trying to have a better understanding whether it’s true what they think that ME/CFS is nothing other than a viral brain inflammation. HHV-6b is wide spread (90% of population have it) and commonly not understood to cause problems expect in immunosuppressed patients for example with Aids or those undergoing organ transplantation. Interestingly enough, virologists who are interested in stopping people dying from such a brain inflammation during transplantation know that a predictor of HHV-6b brain inflammation is low T-cells of the same type that pops up in ME/CFS research everywhere (Maureen Hansen ect.)
Thanks for that excellent reply Lina. Much appreciated. 🙂
Thank you for your kind feedback!
Never mind those of us who have remnants of HHV-6, Lyme, EBV, HSV, CMV, and Micoplasma pneumonias.
Thanks, Cort.
Exactly, “what about bringing down the reactive oxygen species levels in the meantime by calming our immune cells?”
When cytokines (such as CD8) are activated during an infection, they will not stop and can mistakenly attack healthy cells, leading to apoptosis (cell death) and generating debris that macrophages struggle to eliminate.
A somewhat maybe totally off question: Immune system – consistent with both infection an others stressors such as exhaustion/stress disorders right?
And mastcells is one component in the innate immune system, also common with EDS, long covid, ME/CFS, lipedema(?) is MCAS?
Is these findings in line with MCAS findings or mastcells at all? Or which cells are studied in these studies?
This study did not include mast cells. While mast cells activation is a major focus of many ME/CFS experts treatment plans they’ve hardly been studied in ME/CFS yet – but the whole idea of mast cell activation – immune cells that are abnormally activated – seems to fit with this study to me.
Oh no not oxidative stress again! Wasn’t that done to death 15 years ago?
Sorry to be so cynical. It’s probably unfair. Oxidative stress and faulty mitochondria have certainly been smoking pistols for a while. Maybe they were close before but are now getting hotter?
For me the neuroimmune hypothesis’s remain the most compelling
🙂 I know – I’ve felt the same way about oxidative stress. It’s always seemed so nebulous but I like the oxidative stress mitochondrial immune cell connection. It’s going to be interesting.
Exciting research! Thank you, Cort.
Love that this young PHD student came along with new eyes and a fresh perspective. And thank you for putting the Gist first. Actually made me want to take the time and energy to read the whole article! 😄
Yah to Shankar for his persistence! Glad the GIST worked in its new place – someone suggested putting it there. I think its a good idea.
OMG Cort, we keep going in circles -:) it makes me dizzy (lol)
But there is more clarity about which systems are disrupted in ME/CFS. It is now described as a multisystem disease. How can we solve the puzzle?
Perhaps we as patients can create a model where the pieces fits together with substantiated objective literature. In the simplest possible way.
There is no lack of knowledge or intelligence here on the forum 🙂
CNS-ANS-HPA-as-immune- and energy system….
I was thinking the same thing :). What will connect all these systems? I think the long COVID researchers are kind of dazzled by the complexity of it. They keep saying – I’ve never had to work with X or Y or Z field before.
I’m thinking this is a perfect disease for AI. Plucking out the key factors in very complex systems that humans don’t have the brainpower to analyze is, after all, what it does.
New study brainstem involved again 🙂
https://www.frontiersin.org/journals/neuroscience/articles/10.3389/fnins.2023.1318094/full
Ah – the brainstem again! I hope RECOVER and long COVID researchers are watching this. Brainstem problems could be producing so much….
Interestingly, CCI relates directly to the brain stem and its ability to keep the most vital systems and sub systems functioning to maintain their functional roles.
One of the issues that needs to be looked into is having to thick of blood, alot of people die from this and nothing is said, it causes heart attacks and is now being associated with long covid, my 2 cents.
Hi Gijs, here is my understanding of the problem. I tried to explain it to you before. I am not sure what it is that you won’t give the HHV-6b hypothesis any credit? Because there is no other hypothesis equally well supported in ME/CFS than this one.
Here again my synthesis:
The most advanced and supported hyothesis to the question what is the immune system fighting against is that ME/CFS is HHV-6b brain inflammation. It is supposed that there is an initial problem with the immune system after an infection (a specific type of T-cells is at the center of that research) which then allows HHV-6b to wake up and do damage to the brain, the vessels, the mitochondria and the immune system.
Bupesh Prusty together with Mark Williams and Maria Ariza and others were able to come forward with this theory and have beautiful research results. The most interesting ones are a study that shows how enzymes that get produced early on in the HVV-6b reproduction process hamper and even may destroy proper mitochondrial functioning. Link: https://pubmed.ncbi.nlm.nih.gov/32327453/
Then a study by Prof. Jacqueline Cliff of Brunel University in London who was able to show with her team that there was indeed HHV-6b to be found not in the blood(!) but in the saliva of ME/CFS patients and that the virus load correlated to symptom severity (it makes a lot of sense that it would show up rather in the saliva than in the blood given that as far as I know herpes transmission route is smear infection).
However, they could not decide whether HHV-6b was the driving pathomechanism of ME/CFS or a consequence of a yet unknown more foundational problem.
After that came again a study by Bupesh Prusty and colleagues where they were able to detect the typical m-RNA mix that is produced in HHV-6b reproduction in brain tissue of patients who have died and had ME/CFS: https://pubmed.ncbi.nlm.nih.gov/36589231/
Currently I am awaiting eagerly the results of a new and pretty big and carefully designed study by Prof. Cliff and her colleagues: https://www.brunel.ac.uk/research/projects/reactivation-of-herpesviruses-in-chronic-fatigue-syndrome
Here they are now trying to have a better understanding whether it’s true what they think that ME/CFS is nothing other than a viral brain inflammation. HHV-6b is wide spread (90% of population have it) and commonly not understood to cause problems expect in immunosuppressed patients for example with Aids or those undergoing organ transplantation. Interestingly enough, virologists who are interested in stopping people dying from such a brain inflammation during transplantation know that a predictor of HHV-6b brain inflammation is low T-cells of the same type that pops up in ME/CFS research everywhere (Maureen Hansen ect.)
Hi Lina, Thank you for your extensive and well-substantiated response.
I am aware of this hypothesis and certainly do not reject it. But I also remain open to other hypotheses. I do wonder what role the intestines play in this. As you know, human herpesviruses can also function as co-pathogens (Munawwar and Singh 2016). Precisely this makes it difficult to determine at this time whether there is a causal relationship.
After transmission, HHV-6A and HHV-6B both preferentially infect CD4+ T lymphocytes. CD8+ T lymphocytes, B lymphocytes and other cell lines are also infected, such as blood and bone marrow mononuclear cells, endothelium, epithelium and cells of neural origin. This makes it an interesting candidate.
But why can different types of infections and stress lead to an ME-like illness? Is it a combination of pathogens? Or a problem in the stress system? I look forward to further research.
So, as I understand it, sorry brain fog is bad today.
The immune system is overactive and is killing the mitochondria. All this oxidative gunk is building up.
Therefore, we need to calm down the immune system and clean out the gunk. Hoping that the initial viral infection doesn’t run wild while the immune system is rebooting.
My question: does anything besides steroids, shut down the immune system, or do we need to discover a new drug?
Prolonged use seems to be problematic for most people.
Effective antioxidants would help – some people do do better on Metformin and/or NAC and better ones are being developed. If an infection is present finding a way to clear that using monoclonal antibodies or antivirals would be really important as would boosting the immune system. Finding ways to support the mitochondria would be another possibility
DMARDs (disease-modifying anti-rheumatic drugs) and biologics reduce or modulate immune function.
Some articles consider biologics to be a sub-category of DMARDs, and divide the medications into traditional and biologic DMARDs.
They all come with risks, of course. Some are less risky than steroids though.
I have been searching for a medication or supplement to down regulate my overactive TH2 cells for a long time ,but can’t find anything. I am quiet sensitive to any toxins being moblized, so it would have to be something that wouldn’t cause a detox reaction.
I can really recommend to give mindfulness meditation, yoga, shiatsu and acupunture a shot. If you’re well enough also some physical exercise below your energy limit.
It’s cheap, it won’t do any harm. It supports the body’s natural ability to heal. I have fantastic results. I was moderately affected and had to stop trying to work again three years ago. I am now without a relapse (crash) for 14 months and slowly recovering with mainly repeating everyday the things that I listed above. It feels a bit like being a tuberculosis patient at the beginning of the last century going to Davos or the Mediterrenean to rest and recover.
“Could this increased T-cell proliferation combined with reduced energy production be another version of the “wired but tired” processes we’ve seen where systems that are always engaged and never rest poop out quickly when called to respond?”
I’ll give you another one, potentially: Maybe the mitochondria themselves….They observe that in physical exercise that triggers PEM the normal hypermetabolic reaction is paired with a decline in hypoxanthine as usual, but in the presentation of PEM this is followed by markers of hypoacetylation, an event known to be related to mitochondrial damage.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6787670/
Nice – another way for exercise to whack the mitochondria and produce PEM 🙂
Has anyone tried giving people with CFS a short course of steroids while measuring some of the parameters menetioned above?
Or maybe it would be difficult to do such a study, as even short courses of steroids can do damage, and it wouldn’t receive ethical approval.
Nancy Klimas has been talking about something like that for, I don’t know, 7 or 8 years? And we never seem to hear anything. So obviously not promising
I have heard of people with ME/CFS doing quite well with steroids – the problem is using them long term. It just doesn’t seem feasible – but a short-term study which identified what the steroids were doing in ME/CFS to be helpful – that sounds like a great study 🙂
I second that I become a normal person on steroids. And then dip into hell with every tissue in my body on fire when they wear off.
Does anyone know of any medications or supplements that can down regulate TH2 cells?
If I remember correctly Pine extract which is used to shift from th2 to th1 and which is also used in CFS. I tried in 2002, did seem to help some. I do not remember the exact name of the product. They were drops. Something to do with pine though.
Taurine usually helps me a lot with allergies, I also get a sense of well-being and calmness. It’s a conditional aminoacid used by the immune system(?)/body in times of stress events. It also helps to maintain cell membranes. Maybe worth trying?
So frustrating that instead of looking to identify and eliminate the root cause – pathogens – we are focusing on tamping down the immune system. That seems like a disaster in the making, when you are fighting multiple infections. There is no common sense left in this world, apparently. So disheartening that the NIH is not going to fund studies related to pathogens (so I thought I read in another blog). Criminal, really.
I agree Linda.
Since Long Covid is looking so much like Chronic Lyme and ME/CFS, I suspect those who fail to recover from Covid (and Lyme) already happen to be harboring what I personally call “the Long Key Agent”, whether that be Borellia burgdorferi or some similar spirochete, or what Willie Burgdorferi referred to as “The Swiss Agent” (likely a Rickettsia like Bartonella,) or some combination of several coinfections including a virus, or one as-yet unidentified culprit.
I find it ironic that NIH dished out millions (billions?) over the years to study amyloid plaques in Alzheimer’s brains and how to get rid of them. Turns out the plaques were PROTECTING the brain tissue from the very pathogens that were destroying the brain. Alan MacDonald discovered parasite worms full of Lyme bacteria inside plaques within Alzheimer’s brains.
We need more focus on the root cause of the problem! (Not to disparage any of these researchers who are pursuing other avenues. We applaud all their efforts to help us too!)
Thank you for the validation, Julie! We need a major paradigm shift in the way of thinking in the medical research community and government agencies concerning these chronic diseases which have overtaken so many.
Well, if you look at the history of disease and disease “treatment”.
“They” havnt found a cure for very many diseases, and it’s doesn’t seem to be getting any better.
Look at Elaine gotchal(spelling?)….
She cured her daughter of chrones disease by a 7 yr.diet .
She thought she would go tell all the gastro world…they ignored her completely.
How about this fairly recent M.S. person(a dr. Non the less) that cured her m.s. by diet and other interventions. Not one researcher looking into her case.
Seems to me that if they can’t develope an $expensive$ drug ,then sell it…only as a “treatment”….they never want to hear of any cure.
I guarantee the NIH will never find the science and cure for this disease.
It’ll be private funding that will.
I have a sister in law that has progressive m.s.
I swear that there isn’t one tiny bit of positivity in her attitude. It’s all, pity me,all of the time.
I told her one day soon they will be able to cure her m.s. She got very upset and said she “didn’t want to go back to work” and that she “liked her life the way it is”
“They” told her to start having these injections that will stop her ms progression its tracks.
It costs the govt🇨🇦 $2000 a pop….it hasn’t done a dam thing.in fact, she’s gotten a lot worse….but..,she keeps going for the shots.
We see this with seniors a lot….loaded up on way too many pills $$$
Don’t talk to me about old folks homes and donuts for breakfast, dinner, and supper😉
At one point- quite a while ago – the NIH said they would not fund pathogen studies in ME/CFS. I don’t know if that’s still true or not but now long COVID is producing a lot of pathogen studies and I imagine the NIH is funding some of them. We’re going to learn a lot about pathogens over the next couple of years. As I was told Nancy Klimas said “Pathogens are back”! I hope so.
Thank you, Cort, that is encouraging.
Yawn
Pathogens and ME/CFS are soooo 2007
😂
Matthias, the field of virology does not look the same as 2007. They have technologically advanced. When an older generation of researchers in about 2010 gave up on pathogens and especially herpes viruses their decision was based on the insight they had gained with those former lab technology. Maria Ariza in her “The Herpes Viruses Are Back!” : ) overview from January 2021 of the new promising findings gained after 2010 explains this there.
https://pubmed.ncbi.nlm.nih.gov/33572802/
I would wager viral factors are not perpetuating the illness!
Time will tell I guess, although we’ve had 25-30 years so far. Perhaps you will be right, with new technological approaches
Don’t wager, Matthias! Go read the studies that I have provided the links to yourself and update yourself on the current research status.
HHV-6b brain inflammation is the best established theory to explain ME/CFS that currently exists.
The fact that you find it improbable doesn’t make all the evidence that has already piled up less convincing.
Assuming this theory is proven correct – and that is far from certain, when are the study results due? -, what’s the pathway to treatment?
It’ll of course take time to have more studies and then have them replicated.
As I have mentioned before, I was prescribed aciclovir on speck because we suspected a HHV-1 Mollaret meningitis. As a matter of fact it has also good efficacy against HHV-6. There is another similar anti-viral ganciclovir. I was with an active inflammation (crash, ongoing PEM) at that time for more than two months and it stopped within one hour after being on the drug.
They have been known in the ME/CFS community to be helpful for decades.
I thinkt that the mistake people made with those was that they expected them to cure ME/CFS. But if HHV-6b hypothesis is true then the ME/CFS has just the ordinary and typical relapsing/remitting character of herpes virus illnesses like cold sores, genital HHV-2 reactivation, Mollaret meningitis ect. and the drug can only stop the inflammation process but not the damage to the different systems of the body that are afflicted by ME-inflammation, like the immune cells, endothelial cells, mitochondria and especially the brain.
These type of herpes viruses always wake up again when the immune system is compromised. With other words: Only when there will be totally new drugs to treat retroviruses we can stop to pace. : )
On the website of the ME association it is said that the NIH is funding the big HHV-6b study in ME/CFS run by Prof. Jacqueline Cliff, Brunel University, and Dr. Eliana Lacerda, London School of Tropical Medicine.
https://meassociation.org.uk/2022/11/reactivation-of-herpesvirus-infection-in-me-cfs-and-long-covid/
I also think that that is frustrating, given that especially the herpes viruses are back in the game. At the same time I am positive that this will change because in the meantime there are good immunological studies where the researchers hypothesise in the end of the paper that the trigger to all the immune upheavel might be a pathogen, likely herpes : )
The root cause of the situation that we have so few research into pathogens I suppose is that virologists and especially neurologists and virologists if at all stopped too soon to look into ME/CFS and the authorities in the field decided that it had nothing to do with their field.
Thanks god the immunologists have taken over. But since the viruses ect. are not their field they can’t go any further.
Maybe it will take another generation until neurologists and infectiologists are back researching ME/CFS. Because it’s mainly them I think who have most strongly propagated the psychosomatist theory of ME/CFS and are hence responsible for the psychiatric horror that so many people have endured.
Means they will not change their minds to acknowledge their blunder.
Does HBOT reduce or create oxidative stress? Nancy Klimas is adding 2 hyperbaric oxygen chambers at her Institute. But I read elsewhere I that it can create oxidative stress.
It ss very disturbing when the NIH has made commitments to aid and support the most important steps toward identifying and focusing on the Bio Markers that contribute to this life-altering condition. Bureaucracy and its pathways seem to have put the significance of this condition in a pigeon hole, while Drs Ron Davis and others dedicate their lives to identify and implement protocols toward resolving the forefront symptomology.
Vampires and Zombies at the same party?
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10295026/#:~:text=Oxidative%20stress%2C%20a%20condition%20caused,common%20driver%20of%20cellular%20senescence.
Iron! Very interesting. Thanks 🙂
So is there an implication here of the need for increased focus on managing senescence?
I realize everyone is different and I agree with something I read yesterday that it is likely that ME and CFS are 2 entirely different conditions. I would fall on the ME side.
But the whole concept of senescence has really resonated with me. Add to that the fact that for over 3 years all my blood work showed me to have a high red blood cell count that no one has bothered to mention/address.
All factors considered, yesterday I went to donate blood. I can definitely tell the difference today. I’ve already scheduled my next donation to force my body to make new healthy cells.
Recommended by my nutritionist for weight issues (my cortisol is too high and I was gaining weight doing intermittent fasting and carnivore), I’m excited to start Prolon next month to further directly address scenescent management (fasting-mimicking protocol to eradicate zombie cells).
I have always believed my issues were cellular (OAT showed a breakdown in the Krebs cycle which no one has addressed). But who’s going to help with cellular issues?
Finally feeling hopeful.
I was watching a guy on YouTube the other day talking about Long Covid and he was saying that even having red blood cell counts that are within your guidelines your blood can be to thick and cause you to have all kinds of issues including Heart attacks, he said its a good idea to donate blood which thins your blood out and you will feel better.
Interesting. We’ll see how it goes. I’m on vacation so schedules are off, but for now I’m going to bed way earlier, getting more sleep and lasting longer through the day.
I’ve been donating blood because my ferritin became soooo high.
My toenails that I kept ramming into the dishwasher, baseboards etc.had died and stopped growing for at least 10 yrs, are now growing again since giving blood.
But….I’ve added a couple other changes as well…like herbal and NAC.
that’s my biggest problem, I never do things one at a time…I bet it’s the giving of blood because my ferritin has come down to very close to normal now.
As someone who’s 10+ years battle with chronic fatigue started due to using metformin, I personally don’t believe metformin is the miracle drug it is believe to be and a lot more research needs to be done to understand how it effects the body.
I took metformin for PCOS and spent over 9 months being sent to doctors to try and figure out why I had suddenly suffered from fatigue, while every doctor believed metformin couldn’t cause fatigue. After no answers from my doctors I decided to stop taking metformin and see what happens. I recovered to about 70% of my previous energy within days of going off of metformin, and only now (10years later) I understand that I was still suffering from PEM after I stopped taking it. A year after I had stopped taking metformin I had moved to a new area, found a way to balance my PEM and found a new doctor, who questioned my theory of metformin and asked me if I would try the medicine again. I foolishly agreed thinking my doctor might know something I don’t. In the 4 days I was taking metformin I went from a functioning being (adjusting to the 70% I was functioning) to a non-functioning being in a full blown fatigue flare up, with very little ability to care for myself or my children. I stopped taking the metformin after 4 days and I have spent the last 9 years working tirelessly, trying to recover (at least back to my 70%) from that 4 day experience.
I will never be able to prove that metformin gave me chronic fatigue! I continue to fight for disability and try to prove that I don’t have the ability to function as one should and I can’t work. Yet, each day I try an improve my health in any way, so one day I can enjoy some level of a quality life…
There are people out in the world suffering from long term chronic fatigue due to metformin and the medical community does nothing about it, because there isn’t enough scientific evidence to prove it.
Thanks Erinn for relaying your story. Our bodies are complicated things, for sure! Good luck with your disability.
As a 40 yr sufferer, you’ll soon learn what to say,when to say it….when to go to them, and when not to.ive almost stopped going to them for my me/cfs.
Up here in can can 🇨🇦 land they just see you for an appointment(s).the doctor makes a living, and a good living, but the patient remains the same.
And if you don’t follow their drug forcing and refuse to take a drug, you become on the bad side of them and now I see they have included patient firing as a new way to flush you.
It’s next to imposible find a family dr. In Canada since the system was broken and way more broken since they’ve let millions of others immigrate. BIG trouble in the TRUE DOLT arena. But, it’s free entertainment just like watching donald
Metformin can cause mitochondrial dysfunction resulting in lactic acidosis. It shares the same transporter as thiamine. I would try high dose thiamine
I think this is most descriptive situation yet. Its starting to come all together. The trigger causing oxidative stress maybe for some is a defect in glutathione production… which is actually needed for T cell function. Then along comes herpesviral activation spreading from nerves to neurons and causing microglial activation. And EBV infects the natural killer cells further impairing the immune system. Mast cells are chronically activated since the big guns are not functioning. I find glutathione as a supplement doesn’t work too well and taking an IV can cause much worse problems as it can turn into oxidized glutathione. We need recycling factors with the glutathione. Currently trying glutathione injections with B2 and carnitine. And taking other vitamins needed as a supplement. I will report how it goes. I am taking valcyclovir too but its causing some issues as it inhibits mt DNA polymerase. I need to take other meds with it that stop herpes replication like anything that slows down calcium influx into the cell as herpes uses calcium to infect neurons. Spironolactone, memamtine and t type calcium channel blockers might help. And of course stay away from anything that increases adrenaline or steroids or nicotine. It will increase cox 2 and replication. Very hard to get a doctor to try out these things though.
Also I am very curious why it is that they don’t want to use antivirals or do proper DNA testing on people. Herpes encephalitis should be in their medical literature. Is it only recoginized when you are on death’s door? Something weird is going on.
Herpesvirus PCR DNA testing I mean. The only testing I can get is if I had herpesvirus in the past. I can’t even get a test to show if I have active markers.