
Thanks to Geoff for providing a narration of this blog
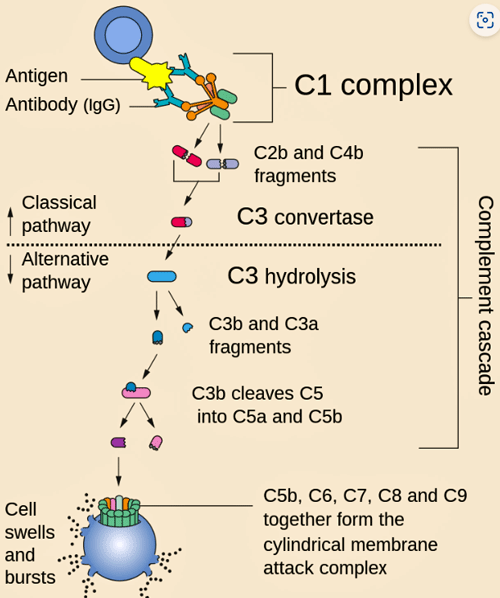
The complement cascade – it starts with an antigen (foreign substance) and ends with the membrane attack complex that drills a hole in the invader.
The “complement” system doesn’t at first glance sound like much. Its name reflects the fact that, in part, it “complements” the immune system; i.e. it triggers immune cells to attack pathogens and clean up damaged cells. It also, though, torpedoes bacteria by drilling a hole into them, causing them to spill their guts.
All in all, the rather unimpressive named complement system plays a major role in the innate, or early, immune response – which is responsible for a great deal of inflammation in the body. A breakdown in the complement system can produce many problems including inflammation, or an inability to fight off pathogens.
Indeed, dysregulated complement systems have been found to play a role in many diseases including lupus, rheumatoid arthritis, asthma, multiple sclerosis, vasculitis, ischemia-reperfusion injury, and others. Overactivated complement systems can cause tissue damage while underactivated ones can leave one susceptible to infections.
The complement system consists of about 50 proteins and protein fragments that get activated when they bump into signs of infection and/or damage. One arm of the complement system gets activated when it comes across antigen-antibody complexes (when an antibody binds to a toxin or foreign substance – classical pathway); another when the proteins meet up with sugars produced by bacteria (lectin pathway); or when a cell come across microbes (alternative pathway).
Once activated, complement factors trigger the production of cytokines, which then trigger immune cells to attack pathogens, clear the body of damaged cells, and produce inflammation.
The complement system – which sits at the base of the innate immune response – is complex! (Image of classical complement pathways by Architha-Srinivasan CC-3, Wikimedia Commons)

THE GIST
Instead of directly killing pathogens for cleaning up broken down cells, the complement triggers immune cells to do that. It’s no small player, though. A critical part of the early or innate immune response, the complement system plays an important role in inflammation, and has been found dysregulated in many autoimmune and other diseases.
The 152-person study did an open-ended analysis of a large number of proteins (<7,000). A pathway analysis found the complement pathway was highly activated in the long-COVID patients.
Next, a machine learning (AI-type analysis) plucked out proteins associated with the complement system in the long-COVID patients.
- An upregulation of early complement factors and the downregulation of the later complement factors suggested that the system was producing inflammation and not effectively fighting off pathogens.
- Speaking of pathogens, elevated levels of both anti-CMV and anti-EBV IgG titers in long-COVID patients at 6 months suggested that a herpesvirus reactivation could be driving the complement system activation.
- Not only that, elevated coagulation factors suggested that microclots could either be driving the complement system activation, or might be driven by it.
- Plus, some evidence suggested that monocytes – a key part of the complement cascade – were also dysregulated. Monocytes have shown up big time in some recent ME/CFS studies.
- All in all, the study produced a nice package – complement activation associated with herpesvirus activation, coagulation, and monocytes – each of which have been found in ME/CFS.
- Since this study was published, two other studies have highlighted the complement system in long COVID.
- While the complement system has never been a major focus in ME/CFS, several studies have found evidence of complement dysregulation, including one which found complement abnormalities after exercise.
- Complement dysregulation plays a major role in many diseases, and a variety of drugs have been produced to battle it. The authors of the various articles proposed almost a dozen drugs that could be piloted in long COVID and ultimately, possibly ME/CFS.
“C1q binds directly to the surface of the pathogen. Such binding leads to conformational changes in the C1q molecule, which leads to the activation of two C1r molecules. C1r is a serine protease. They then cleave C1s (another serine protease). The C1r2s2 component now splits C4 and then C2, producing C4a, C4b, C2a, and C2b (historically, the larger fragment of C2 was called C2a but is now referred to as C2b). C4b and C2b bind to form the classical pathway C3-convertase (C4b2b complex), which promotes cleavage of C3 into C3a and C3b. C3b later joins with C4b2b to make C5 convertase (C4b2b3b complex).
It’s no wonder the finding brought an, “Oh no! Not the complement cascade”, from Eric Topol as he mused on the recent complement findings in long COVID.
While each of the three pathways are initiated in different ways, they all end up cleaving complement factor 5 (C5) into C5b when then binds to C6, C7, C8, and C9, to form the terminal complement complex (or TCC; C5b-9). This complex bores holes into the membranes covering pathogens, causing the internal components of the pathogen to leak out and die.
The Study
This Swiss study, “Persistent complement dysregulation with signs of thromboinflammation in active Long Covid“, made waves when published earlier this year for several reasons. For one, it was pretty comprehensive, and for another, it suggested that complement problems in long COVID may be associated with two other big issues – coagulation and herpesvirus activation.
The 152-person study did an open-ended analysis of a large number of proteins (<7,000); i.e. it statistically assessed all the proteins it found to see what insights would emerge.
The pathway analysis examined all the biological pathways found in the proteins that were differently expressed in the long-COVID patients. Think of it a large overview of what got tweaked biologically in them.
Filling two of the top three spots (“complement cascade,” “regulation of complement cascade,” and “immune system”), complement pathways dominated the protein findings.
The fact that the complement dysregulation showed up during the initial infection in the long-COVID patients (but not the recovered patients) suggested that problems with the complement system may lay the groundwork for long COVID.
An analysis of the proteins themselves found that complement c7 was significantly decreased at 6 months in the long-COVID patients. Next, a machine learning (AI-type analysis) concluded that proteins associated with the complement system were the most dysregulated six and 12 months after infection in long COVID.
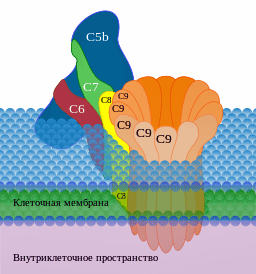
High levels of earlier complement factors plus low levels of membrane attack factors indicated the complement system was off. (Image by Д.Ильин,-CC0,-via-Wikimedia-Commons)
Reduced levels of the soluble complexes (C5b-7, C5b-8, and C5b-9) that are associated with the later “terminal complement complex (TCC)” were found. The later terminal complex consists of complement factors that get deposited onto pathogens in order to kill them. At the same time, the long-COVID patients exhibited an increase in early TCC factors (C5bC6).
The upregulation of early complement factors and the downregulation of the later complement factors suggested that something had gone awry. The authors assessed protein clusters associated with complement factor c7 in an attempt to figure out where the breakdown occurred. (C7 is the factor that allows the C5b-7 complex to integrate into cell membranes of pathogens.)
High levels of the C5bC6 complex that preceded it indicated that the complement system was likely hyperactivated, causing inflammation. Indeed, the increased levels of two different complement factors (C2, Ba) suggested that two of the three arms of the complement system had been overactivated in the long-COVID patients.
Herpesvirus Reactivation (again)
The researchers uncovered two possible reasons why: elevated levels of both anti-CMV and anti-EBV IgG titers in the long-COVID patients at 6 months could be triggering the complement system activation.
Coagulation (again)
The complement and coagulation systems are closely linked. Because complement can also be activated directly via a coagulation factor called thrombin, a coagulation cascade could also explain the complement activity.
The findings suggested that complement activation could be producing blood clots, or vice versa!
indeed, elevated pro-coagulation factors (vWF and TSP-1) and decreased anti-coagulation factors (ADAMTS13, PAF-AH, and ApoA1) indicated that a “thromboinflammatory” response was present; i.e., a coagulation-activated inflammatory response was present in the long-COVID patients. Evidence of a “hemolytic process” in which red blood cells are being destroyed also showed up.
The authors noted that complement activity has been associated with the microclots found in long COVID before, and complement deposition has been found on the endothelial cells lining the blood vessels and platelets in autopsies of long-COVID patients.
Monocytes (Again)
Monocytes showed up big time in a recent ME/CFS gene expression study. In fact, that study suggested they might be ground zero for the immune problems in ME/CFS. That’s an interesting finding given that monocytes play a crucial and multifaceted role in the complement system.
The long-COVID study, interestingly, found that the monocytes showed “distinct transcriptomic changes”; i.e. distinct changes in gene expression – as did the ME/CFS study.
Filling the Gaps?
This study’s ability to potentially bring together important factors (complement activation, herpesvirus activation, coagulation triggered inflammation, monocyte dysregulation) that could combine to produce the chronic disease state we know as long COVID was impressive. Plus, it may also have resulted in a diagnostic signature as well.
One of the co-authors, Dr. Felicity Liew, stated, “It is unusual to find evidence of ongoing complement activation several months after acute infection has resolved, suggesting that long COVID symptoms are a result of active inflammation.”
Reviewing the study, Dr. Russo agreed that the activation of complement in long COVID could be producing the microclots found, which could cause “premature cardiac events, dementia, respiratory failure, and renal failure” as well as the fatigue that people with long COVID struggle with.
The authors proposed using antivirals to target coronavirus and herpesvirus infections and other drugs (anakinra, JAK inhibitors) to target the overactive complement system in long COVID “… and possibly other postinfection syndromes.
Complimenting the Complement Findings
Since the paper was published, two more studies have highlighted complement activation in long COVID.
British Study
Just last week, a British study, “Large-scale phenotyping of patients with long COVID post-hospitalization reveals mechanistic subtypes of disease“, involving dozens of researchers, found evidence of complement activation in formerly hospitalized COVID-19 patients who had come down with long COVID.
Monocytes – which play a critical role in the complement system – showed up in 2 long COVID studies as well as recent ME/CFS studies.
The study – which also assessed plasma proteins – also found evidence of monocyte/macrophage activation and, in a nice twist, was able to link immune factors with symptoms.
One set of factors associated with cognitive problems (brain fog) suggested that neuroinflammation was the cause. Elevations of another protein also found in irritable bowel syndrome were associated with gut issues. Proinflammatory signatures dominated the cardiorespiratory, fatigue, and anxiety/depression groups. Markers of myeloid inflammation were associated with fatigue.
These factors are important because they could provide treatment approaches for these different symptoms.
The authors noted that all the mechanisms proposed for long COVID (autoimmunity, thrombosis, vascular dysfunction, SARS-CoV-2 persistence, and latent virus reactivation) potentially involve the complement system and myeloid inflammation. Myeloid inflammation involves monocytes, macrophages, dendritic cells, and other cells of the innate immune system that the complement system interacts with.
They proposed that trials of steroids, IL-1 antagonists, JAK inhibitors, naltrexone, and colchicine be done.
Welsh Study
Plus, a preprint out of the University of Wales had the enticing title, “Complement dysregulation is a predictive and therapeutically amenable feature of long COVID“.
This 243-person study, which used Elisa assays, uncovered markers of significantly increased activation in all three complement pathways (classical, alternative, terminal) in the long-COVID patients (compared to recovered COVID-19 patients).
An AUC score of .785, using four complement factors, indicated that those four factors did a “good” job of separating long-COVID patients from healthy controls.
The researchers proposed that pilot studies assessing three drugs: pegcetacoplan (targeting C3), iptacopan (targeting FB), and vemircopan (targeting FD) be undertaken in long COVID.
The Complement System in Chronic Fatigue Syndrome (ME/CFS)

Exercise-induced complementation activation in ME/CFS.
While the complement system has never been a major thrust of immune research, it has shown up several times. The interest began when a 2003 CDC study found increased levels of a complement factor called c4a in people with ME/CFS after exercise. A small CDC 2009 gene expression exercise study concluded that exercise might have altered the lectin pathway, resulting in a downregulation of protease which then boosted C4a levels and produced inflammation.
In 2015, another CDC gene expression study highlighted alterations in two complement genes that were associated with physical fatigue, body pain, and overall ME/CFS symptom scores.
In 2019, the Nijs group found that increased levels of C4a after exercise were associated in increased pain sensitivity in one measure (but not two others) in ME/CFS. Then in 2021, a different complement factor (C1q) “unexpectedly”, according to the authors, showed up in a large ME/CFS study. The study, which assessed just 4 complement factors (C1 inhibitor, C1q, C3, C4) used more or less common blood tests. ME/CFS patients with high C1q levels also had significantly increased C3 and C4 levels, and reduced C1 inhibitors.
The authors suggested that chronic activation of the classical complement pathway might result from an infection or from the clearing away of damaged cells in a large subset of patients, and proposed, interestingly enough, that coagulation be studied in this group.
Treatment Potential
Given the role complement plays in many autoimmune diseases, it’s not surprising that a variety of drugs have been approved to treat complement dysregulation. That’s potentially good news if complement should turn out to play a major role in these diseases. They include:
- C1 inhibitors: Berinert, Cinryze, Ruconest, and Enjaymo (sutimlimab)
- C3 inhibitor: Empaveli (pegcetacoplan), SYFOVRE™ (pegcetacoplan injection)
- C5 inhibitors: Zilbrysq (Zilucoplan), Veopoz (Pozelimab), Izervay (avacincaptad pegol), Soliris (Eculizumab), Ultomiris (ravulizumab), and Tavneos (avacopan)1
The authors of the long-COVID and ME/CFS studies proposed that clinical trials of the following drugs be attempted: pegcetacoplan (targeting C3), iptacopan (targeting FB), vemircopan (targeting FD) anakinra, JAK inhibitors, steroids, IL-1 antagonists, naltrexone and colchicine). (Note that a Dutch anakinra trial did not find that anakinra reduced fatigue in ME/CFS.)
Conclusions
More work needs to be done, but it was good to see the complement system show up big time in three recent long-COVID studies. All found evidence of complement upregulation, two found evidence of monocyte issues – a potentially important topic in long COVID and ME/CFS – and the biggest study linked complement upregulation to coagulation – a potentially very valuable tie-in. As other long-COVID and ME/CFS studies have done, these studies once again pointed fingers at the innate immune system which is responsible for much inflammation in the body.
While the ME/CFS studies found different dysregulations, the association of complement activation with exercise was intriguing, and one study pointed to possible coagulation issues in ME/CFS. A gene expression and a gene polymorphism study highlighted the complement system in ME/CFS well, and recent ME/CFS studies have highlighted monocytes – key players in the complement system.
Finally, the authors proposed a variety of drugs we haven’t made much acquaintance with before that might help return the complement system to health and reduce the inflammation they believe is driving these diseases.
Time will tell how this all turns out, but as always, it’s good to see studies pointing arrows at the same culprits.






Ho hum…twittle dee dum, another…
“more work needs to be done”
It gets a little tiring doesn’t it? I’m afraid we’re all going to see more of “more work is needed”! Particularly with these rather new findings “more work is definitely needed”. However, maybe you can take some comfort that the findings appear to be linking together well -suggesting that they’re headed in the right direction.
If it helps, Jarred Younger thinks that their year there will be no “more work is needed” regarding neuroinflammation – it will be a solid fact. I think the same is probably true with herpesvirus reactivation – it’s clearly present and causing problems. Chris Armstrong has stated that its now clear that our cells are using amino acids to fuel themselves rather than better substrates. That may a done deal as well. The NIH made a statement that gut butyrate issues are pretty solid now too. I suspect we’re going to see reduced blood flows to the brain particularly while standing become solid as well.
The problem in ME/CFS is that we haven’t had enough big studies to nail down some of these things – but over time they are slowly getting nailed down.
It seems like every month we’re seeing new treatment possibilities. While it will take a while to get those tested the fact that we’re seeing them bodes well for the future.
If you have ME/CFS I hope that you’re encouraged by the many lines of overlap that are showing up – the herpesvirus activations, the innate immune system involvement, the coagulation, the exercise results, metabolic and gut results…
It’s going to take time, though. That time would be surely shortened if we can get the federal government to fund more long COVID and ME/CFS/FM research and advocates are working on that.
Cort and all readers:
Please take what I say after 4 years of research review beginning with bootstrap learning in functional medicine… seriously. Because I cared about the illogical suffering of one person.
I have observed HeathRising and observed it having its downs…. eg. where it had seemed the NIH would fund a solution… and then the NIH capitulated.
1. The main issue in CFS is low-grade plus flare-up INFLAMMATION.
2. We agree that there is Brain Inflammation.
3. Brain Inflammation can be the cause for all that then is produced in the body.
Why? Because while the body can withstand a temperature flux of 95 to 104,
THE BRAIN CAN ONLY SURVIVE within a +/- 1 degree flux, and an whole degree is very very bad.
4. CFS long haulers on this site, may God bless each and every one, concur that they always knew they had inflammation in their brain. I don’t know how you have been sure, but it is YOUR body, and YOUR reality. YOU know it best.
5. JOB #1 is to control Inflammation. If you do not, then you will not progress.
How? Nature has provided us with foods….. foods…used for centuries in foreign lands…. which are called ADAPTOGENS…. which help the body to MODERATE its fluxuations.
Ashwaghanda is a household name in India, and with Indians come to Canada.
Curcumin counters inflammation and it comes from TURMERIC
TURMERIC is the yellow in curry. Curcumin is an extract.
5 a) What inflammation am I controlling?
Who cares. We don’t know. We only know that if you control it,
your CORTISOL levels can come down.
6. When you calm your body, from OUTSIDE, thank you to the natural world, then the restoration processes can be ALLOWED to begin..
Subset
subset
other subset
boring subset
protectmyself by saying “subset” since I don’t want to give anyone false hope………………………
NO.
There may be DNA subsets of people
PRONE
to DNA manipulation .
by toxins
but INFLAMMATION IS BASIC TO ALL.
1. increased blood flow in a key region
2. swelling (see blood flow)
3. soreness (see increased blow flow causing increased sensitivity)
4. Possible scarring (to IMMOBILIZE the tissue so you CANT do anything
stupid with it.)
5. Did I mention redness??
HULLO !!!!! if you can’t progress past persistent or excessive inflammation,
then why
oh why
would your body allow you to resume full function?
YOU NEED TO:
a) eliminate non-essential inflamers: (potato, tomato, peppers, simple sugars)
b) DETOX carefully as per a professional
c) Give your body nature’s wise provision for stress as mentioned
AFTER THIS begins to restore you, and your sleep,
THEN you can begin to restore your functions in your Adrenal glands which are MAXXED from the constant low-level or flare-up stress.
That last point is an whole new level of change.
My OPINION is this: CFS is temporary. Doctors have failed and failed and failed to
diagnose it
understand all that it entails
treat it in any sensible manner.
Your body is not a MALE doctor from the OLD ENGLISH school of GENERAL practitioners who give themselves 15 minutes to choose a treatment OR DISMISS YOUR SYMPTOMS.
DOCTORS DID NOT CREATE YOU.
DOCTORS WHO DENY THE NATURAL WORLD WILL NOT CURE YOU.
THEY WILL ONLY DELAY THE INEVITABLE.
WE BELIEVE IN SOMETHING GREATER
Christopher, Thank you for this interesting and well thought out information. Food for thought, certainly!
Thank you, did not know about peppers and potato, adding that to my do not eat list!
It’s the nightshade family…at my worst, I would eat one potatoes
Chip and be sick for a week or longer….same with booze…2 drinks and I’d be hungover for a week. It’s like my system can’t recover very quickly
Thank you for your comment.
Question: are sweet potatoes less inflammatory than white potatoes?
In most cases ,yes. Could be a starch issue
Hi Angelika,
thankfully when I researched that for you I found that Sweet Potatoes do not belong to the Solenaceae plant family, which means they not likely to contain solenins.
Solenins are alkoloids which act as natural insecticides during the plant’s growth.
They are the reason you discard green potatoes, and the reason you NEVER eat the foliage of tomatoes or potatoes. Deadly Nightshade has a very high amount of solenin and the alkaloid is simular to poison ivy in the sense that it is oily.
The link at the end is not scholarly, but it is very helpful with do’s and don’t’s.
Strangely, something known to gradually lower elevated cortisol and keep it moderate, is ASHWAGANDHA. And it’s in the nightshade family! Since I believe hypercortisolism from inflammation, followed by hypocortisolism from the Adrenals just not being able to keep up any more
are the cornerstones of CFS fatigue and non-restorative sleep,
I am going to take the advicein the linked article:
Dont forget the anti-cancer effects of lycopene in Tomatoes nor the high Vitamin C in those and red/orange peppers.
But if you abstain for a while from Nightshades… and you improve… then for you they matter. Oh, but did I mention that Tobacco is a nightshade too?
The old school medical establishment says there is no evidence that solenins cause inflammation. But that’s funny, because other sources show they aggravate existing inflammation. (Last straw). And the link explains something VERY important:
Nightshade members can have the effect of increasing leaky gut and exacerbating inflammatory bowel disease. That means an immune response causes the tight-junction cells if the gut to move apart somewhat, allowing not-fully digested particles into the bloodstream anyway.
You must already know that gluten causes this also.
This week I listened to a set of lectures by Dr. Tom O’Bryan whose topic was brain inflammation.
After having listened to lectures by Dr. Anthony Fasano of Italy who was realsearching causes for autism, I looked up Zonulin by itself. It is known to be part of the gut leakage cause, as Fasano said, but it’s not only in the intestinal lining.
It’s also found in the Blood-Brain-Barrier. And if you didn’t know, the BBB uses TJ cells too, but much tighter.
So “Dr. Tom” said naively ‘if gluten can cause leaky gut, and the BBB has zonulin also, does that mean I could have leaky brain too? Then he stopped his naive role play and said “Yes !!!”
It DOES mean that.
Lastly, Dr. Tom said that of all human foods, gluten in wheat
(or just gluten) is the only food yet known to trigger a reaction from a ‘Toll-like receptor’ in the gut whose purpose is to allow through good things but detect harmful microbes. He said that the outside of the gluten closely resembles the outside of some bacteria. That is what triggers the zonulin (and occludin).
I have a friend who I mention to you often. She is 70 and has celiac disease, CFS and Fibromyalgia too. She turned out also to be allergic to Casein in cow milk.
So she did much better without wheat and dairy, as did many, but not all of Fasano’s autistic children.
Chris
https://unboundwellness.com/nightshades/#:~:text=Nightshades%20belong%20to%20the%20Solanaceae,of%20edible%20and%20inedible%20plants.&text=Tomatoes%20(all%20varieties%2C%20and%20tomato,marinara%2C%20ketchup%2C%20etc.)&text=Potatoes%20(white%20and%20red%20potatoes,sweet%20potatoes%20are%20not%20nightshades.)
Thank you very much!
Thankfully I have been on a low histamine diet for years, which excludes nightshades, as well as no grains and no dairy.
Thanks, Christopher. The link below is to a youtube talk by Dr. Mel Hopperman in which she discusses winter and summer immune systems based on her study of traditional Chinese medicine (TCM). The winter immune system doesn’t mount a robust inflammatory response when confronted by threats, viruses, etc.–this corresponds to my personal experience of 45 years of fibromyalgia/brain fog/depression. She theorizes that anti-inflammatories are counter-productive for winter immune systems. Adaptogens should work, but I haven’t found any that continue to work after some initial relief.
https://www.youtube.com/watch?v=RDCfZzL7tyA&list=UULFnQo6oCvS6YuvaablyMT_sw
Potatos and tomatos are not inflammatory in general. I tested them, indeed for me they are extremely healthy. Everyone reacts differently.
Check this out, Cort
Article in Australian Journal of General Practice:
https://www1.racgp.org.au/ajgp/2024/april/long-covid-sufferers-can-take-heart
a copy of which has been sent to all general practitioners in Australia. Quote from this article:
“An encouraging step forward is the recent discovery in a preclinical model of a peptide inhibitor of nuclear angiotensin-converting enzyme 2 that reverses the persistent inflammation driving long COVID, reduces the latent viral reservoir in monocytes/macrophages and is associated with reduced SARS-CoV-2 spike protein expression in monocytes from individuals who have recovered from infection.32 It also enhances immune protection against SARS-CoV-2 infection. Clinical trials are pending.”
The study they are referring to:
https://pubmed.ncbi.nlm.nih.gov/37369668/
A Youtube video by these same researchers:
https://www.youtube.com/watch?v=JTYVd9daLAo
Where to buy this peptide:
https://www.probechem.com/products_NACE2i.aspx
I have sent for some and will by trying it by nasal spray.
Really interesting, Anne! I love that ACE-2 connection. Thanks for passing it on 🙂
Thanks – yes, I have so much low grade inflammation and pain. I also have low levels (but positive) of Rheumatoid Factor, PEM, fasciculations throughout my body (mostly around eyes and hands), internal tremors, fatigue, and more. For quite awhile I thought I might have ME – possibly FM (or maybe both). The one thing that helps me is low dose naltrexone as it helps me sleep and helps greatly with the pain so I can at least move. LDN is thought to help with inflammation – so perhaps that is what is happening with me. I seem to have issues when I eat peppers – not sure about potatoes, but maybe the nightshade vegetables are ones to stay away from since that is what people with rheumatoid arthritis are told to do in order to decrease inflammation. Also, interesting about the reactivating of herpes viruses. I have had multiple bouts of shingles over the past 20 years since my late 20’s.
My alt complement pathway is 227 and i have so much involvement
My pm scl 75 strongly positive
Organ involvement inssussception
Lung disease
Fibrosis
? Encephalitis
Find
I could go on and on and need treatment its been 4 years with no help or treatment
White matter lesions both sides
Phychosis
01639 414135
Really interesting thank you! In a crash so can’t comment more but think these studies are relevant:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7464301/
https://sportsmedicine-open.springeropen.com/articles/10.1186/s40798-024-00681-0#ref-CR23
Complement studies in athletes! Interesting. As I remember ME/CFS patients had increased c4a products after exercise – which if my neglible knowledge of complement suggest to me an inflammatory reaction. These athletes had decreased C3 and C4 levels if I got in right. How we have something else to assess during exercise.
So interesting – I have been in years-long conversation with other long haulers about why so many of us were fit/athletes when we got and stayed sick. Thank you for these links. Did you see the article in WaPo about athletes and POTS?
https://archive.is/2024.04.10-092204/https://www.washingtonpost.com/health/2024/04/10/pots-medical-condition-athletes-covid-pandemic/
Please everyone send feedback on the Long Covid senate funding proposal to please include ME/CFS. Email LongCOVIDComments@help.senate.gov.
More information: https://www.sanders.senate.gov/wp-content/uploads/4.9.2024-Factsheet_The-Long-COVID-Moonshot-Act.pdf
Thanks so much. It’s on my to do list.
Hi Cort,
Thank you for such thorough overviews, it is so helpful! My question is why are we not taking the Herpes suppression drugs that people with active Herpes take to reduce flare ups? I see them promoted for Herpes everywhere. Why would that not help those of us with EBV reactivation? Appreciate any thoughts on this!
Thanks,
Lee
Lee, I took both Valtrex and Valcyte in 2008 under a respected doc’s care. I went from sick but functional, to disabled. Be careful. just my experience, may work for others.
Oh wow, thanks for sharing!
Done!
Yes – I have done this, phone calls and emails, and posting on my social media to try to get others to advocate. We need to make this happen!!
Done 🙂
Good article. I certainly get some hope from these findings, which are coming to quite similar conclusions. And from high quality institutions. I was especially fascinated by the findings of the Cambridge University study, that found ongoing inflammation and high levels of hepcidin (critical to iron metabolism). That sent me down a long rabbithole of learning about ‘the anaemia of inflammation’. Fatigue, exercise intolerance etc etc
As I said the other day, the USA – huge country, and the world’s wealthiest – is being shown up by all these excellent European studies.
My hope is some trials can be urgently advanced to try to respond to the issues uncovered by the research.
ps. You didn’t really mention it Cort, but complement impacts a whole range of things that have been shown to be out of whack in ME/CFS.
The first Complement System flow or schematic diagram is unreadable whether opened in a new window, or clicked, or double-clicked.
It certainly is. I hunted down the original: https://upload.wikimedia.org/wikipedia/commons/6/68/Complement_system.jpg
Thank you Michiel, I appreciate that very much.
Cort,
you inroduced CMV and never defined it. Normally one would say the full words first then put (CMV) in parentheses. Therefore this is not information.
The reaction to ANY “insult” to our healthy body system will begin with INFLAMMATION.
So a complement involving inflammation…. that is backward. Inflammation INVITES the complement. If otherwise, INFLAMMATION WOULD BE THE COMPLEMENT TO:
something like 50 wildcards of which you only described 2. I do not see how this is helpful.
You will not succeed if you always move on to something new and never synthesize what you have already learned.
That is the equivalent of McCarthyism
ahhhh… Ok. Without your help…. Cytomegalovirus. The most detrimental virus in humans…. if… it gets in to start with. Why? Because it is not a biggie…. you aren’t going to die from it…. but it WILL evade your immune system in the mean time and be restoked from time to time. Maybe this virus should be your focus.
Why? Because this fight is all about EBV or others awaking after evasion. We should create an antibody, destroy it, and forget about it.
But here is just one problem: We are quite accustomed to viruses residing in endothelial cells, where, if we are fortunate, they will remain.
However, over 3 years of my studying research and basic medical texts on viruses and the immune system, it has become more and more evident that some viruses will reside in the neurons. In the nerve cells.
I can not possibly say what awakens a nerve cell to excite a dormant virus.
But if, as you have said all along, that EBV will be awakened, or if, as I am aware,
Herpes 2 goes dormant in the nerve cells (STI),
then we need to be concerned about what irritates nerves.
The following is postulation:
The brain tells the nerves to tell the muscles to act,
after the pre-motor cortex sequences a plan. And the sequencing and re-sequencing
will happen in the space of milliseconds, as will the revised plans.
We all know, on this website, that between the command and the action… “lies the shadow” (T.S. Eliot)
Bringing in 50 immune-complements, to me , does not add to the deduction needed. It convolutes it.
You can boot me off your charitable site if you want, but my only goal is to spend the time that you do not necessarily have, or can afford, to thinktank this crap.
I am daily defeating theories.
In my next text, which I hope will be well-received 😉 , I intend to to summarize my latest summation of all of the publishing on HEALTH RISING, in addition to my self-directed logical elimination and redirection of focus. If you then resent me… so what.
Someone somewhere will want to take the risk to heal, and not wait for some drug
Sorry: if we are *UNfortunate they will remain
C’mon, Christopher. Cort is trying to help us, he is ill too. Maybe you’d like a refund (oh wait, I forgot this is free information). Rather, a simple “thank you” might be more appropriate. This is not a paper published in a medical journal. Use google to type in CMV and I’m sure you’d find the information you need.
Hi Linda,
Thank you for defending Cort. I think you are not familiar with my contributions through commenting on blogs and by emailing Cort directly with ideas synthesized from his blog posts plus other papers. He asked me to write a blog in 2022.
I am trying to help ALL of you, and to help people that I know. I can gather how much suffering it is for you and medicine was not doing its part when I joined Healthrising.
Whether it is insane or not, I have been trying to solve ME since 2020 and my comments here are structured to give either missed points or to insist on sense (i dont mean common sense, since it isnt common)
I re-read my comments that provoked you to implore me to be — more understanding.
I do see that I was grumpy that day and that I put things impatiently. I see that I was acting sucky by saying that I had to look up CMV all by myself. But Linda, if you will read you will notice that I DID look it up on my own. Please understand that there are a million abbreviations, so having a theory presented that is based partially on one really does need a definition of the anbreviation.
Does it necessitate me being sucky and impatient? No, youre right. It doesnt.
I’m sorry to you, Linda, to Cort, and to any other who took offense.
For me, I was challenging Cort, whom I admire, to keep out of the rabbit hole. We need to narrow focus not widen it in this case, I think
Thank you for the note, Christopher. Yes, after posting I saw later that you did find the definition for CMV but could not edit my comment. Also see that you try very hard to synthesize a lot of information for us, so I appreciate that. Understood we all have bad days and in our haste don’t always self edit as we should. I just appreciate all Cort does for us and will admit I ‘reacted.’ Anyway I appreciate the “mea culpa” and it seems Cort knows you appreciate him as I am sure many readers appreciate your efforts. Wishing you the best in your research.
Thanks for the explanation, Chris. I too hate having to use up my precious energy on things that should or could have been handled much easier – like having to look up CMV.
McCarthyism! How did I get lapped in with McCarthyism? That’s a new one!
Sorry about the CMV – I really do try to explain the abbreviations but I missed that one. Thanks for the reminder.
According to the Cleveland Clinic “In summary, the complement system is a powerful defense mechanism that helps keep you healthy by targeting invaders, promoting inflammation, and removing harmful substances from your body” – so it can promote inflammation as well.
Hi Cort,
thank you for your reliably good nature. I try not to put comments on blog articles where they are not relevant, but the last email you wrote to me from bounces back my replies.
Please allow me to (for a change) be brief and relate or dissociate major theories one that I have mentioned to you in the past.
1. I noticed in a Naviaux paper that Aldosterone levels were found to be NORMAL in the CFS/ME study patients.
I had surmised that they would be low from Adrenal Fatigue owing to hypercortisolism owing to chronic lower-grade inglammation and brainflammation, as I term it.
That is important because it puts serious doubt over my supposition that a lack of Aldosterone led to a lack of salt which led to a lack of water in the blood (hypovolemia) which would lead to lowered blood pressure and the accompanying lowered CELL volume might account for misshaped cells into which insulin could not attach (see metformin).
However, since covid attacks all smooth muscle cells, most notably the vasculature, that means that microvessels crucial in the kidneys could be incapacitated to properly retain salt. The eyes too have microvessels.
2. The American Sepsis Alliance
https://www.sepsis.org/sepsisand/coronavirus-covid-19/#:~:text=If%20you%20have%20any%20type,emergency%20room%20or%20call%20911.
said:
“Surviving severe COVID-19 means surviving viral sepsis. ”
My idea there was that SEPSIS in that case would be from our own cytokines and in a “storm” situation which was true in those who succumbed, but not necessarily true in those who were asymptomatic or mild, prolonged poisoning from normal cytokines can push the body into hypothermia, and being condistently down a degree or 1,5 can qualify. The patient needs *continued fight by cytokines, but fewer, and can not sustain the high energy required by the fever response.
3. Lately I commented my most vigorously convicted and my latest theory that while the Ischemic Cascade tends to be looked at mostly for its application to stroke, there has never been a reason to consider System-wide Ischemic Cascade.
I know you cited an interview by (Barbara?) of Rob Phair on the Itaconate Shunt hypothesis of his, and my third point here goes a long way to supporting it. He makes a lot of sense. I watched and took notes.
Here are the 16 steps in the cascade, for reference;
https://en.m.wikipedia.org/wiki/Ischemic_cascade
Two critical points:
Phair mentions GABA and GLUTAMATE. In the end of his theory we see the cell using Glutamate to create barely enough ATP since the Glucose aerobic pathway is knocked out, and since the fatty acid ATP generation sees ATP sequestered instead of used. It is sequestered in this way by the Mitochondria.
A year or more ago the concept of Glutamate Excitotoxicity caught my imagination more than any other factor has in CFS/ME. But I did not understand it enough to explore it.
A week or two ago a member of Healthrising said in a comment that she was taking somerhing to improve sleep that was ‘at least addressing the glutamate excitotoxicity.’
To which I sort of answered her:
“the gluto- WHAT????”
Because Rob made the point of covering the brain’s excito/relaxo chemicals, glutamate and GABA, since mitochondria were desperately using glutamate to get one measley ATP from the glutamate substrate while the other ATP was taken back in the GABA shunt.
And in light if just having researched the Ischemic cascade, I could see that too much calcium would be brought into the cell when response to glutamate was incorrect… and that then more glutamate would be brought in in response, in a cyclical fashion.
Please view the steps of the cascade: in which the normal response process to acetylcholine release —
( to open a channel for Calcium and Sodium to influx and potassium (and chlorine?) to outflux) is messed up.
Rob Phair says the cell (mitochondrion) is stuck in an innate-type “immune response, but the question I now have to answer is ‘why?'”
I’m not sure, Cort if this answers “why?” or if it merely elucidates the nature of the problemz
But Systemic Ischemia can certainly describe CFS/ME and most certainly describes long-covid level endothelial damage. And please review or keep your etes open for kidney dysfunction in CFS/ME since a kidney that CAN’T /WON’T retain sodium doesnt *care if aldosterone levels are correct.
Wow, you’ve been busy, Cort. Thanks again for keeping us non-scientists updated on these studies. It’s very encouraging to know that so much research is going on and that some headway is finally being made.
Hey, Cort did you see that Scheibenbogen re-analysed the Swiss results and came to the conclusion that it was all noise?
https://www.medrxiv.org/content/10.1101/2024.03.14.24304224v1
Hmmmm. But two other studies have also shown complement activation
The two other studies didn’t really show the same thing if you read them. Especially the results of the Wales are quite different to the Swiss study. Just because it includes the words “compliment activation” that doesn’t mean the studies actually agree with each other.
I didn’t say they completely agree with each other. But they all showed some evidence of complement activation. Which was the point I made.
Yes evidence of complement activation, but via different pathways. So they don’t really support each other. Would be interesting if Scheibenbogen was to re-analyse them and see if it’s all noise there as well…
The research that Carmen et al cited regarding age and complement found a correlation only in females.
I can’t see in the Swiss study what the gender balance of the study participants was. But if it was roughly 50/50, then the influence of age is significantly, but not totally, reduced in the patient group.
The many ways ME-CFS or long-COVID can manifest in an individual prove multiple approaches to a cure are needed. A treatment that works for patient A may offer little or no help to others. At the same time, Patient B may be helped by something completely different, but that won’t cure ME-CFS (or long-COVID) in someone else. So, what is the answer?
I have read articles where others have stated that any approach to curing post-viral disease must use blended treatments to address the myriad of systemic breakdowns we have seen in the patient. Isn’t this obvious?
It’s not like we haven’t lived through something similar in the recent past. Did we not learn anything from the HIV/AIDS era? It took a concerted effort and a significant amount of funding to reach the answers to a disease that was essentially a death sentence.
Due to funding issues, we must take small steps, but time is running out for far too many people. This is frustrating for the patients and for the people who love and care for them. I watch my wife slowly become weaker and subject to pain and suffering that should not be countenanced.
Cort, thank you for your continuing work and sharing what you learned.
The immune system is ‘switched on’ by infection and vaccination. Apparently a group of people are susceptible to developing ME/CFS/POTS and Long Covid. I would like to know which patients developed Long Covid after infection and which after vaccination. Research shows that the more you vaccinate against Covid, your IgG4 value increases.
There is concern that COVID-19 vaccination per se might contribute to long COVID (Long Vax)?).
(…) Recipients of two or more injections of the mRNA vaccines display a class switch to IgG4 antibodies. Abnormally high levels of IgG4 might cause autoimmune diseases, promote cancer growth, autoimmune myocarditis and other IgG 4-related diseases (IgG4-RD) ”
This is concerning.
https://www1.racgp.org.au/ajgp/2024/april/long-covid-sufferers-can-take-heart?fbclid=IwAR0_LO6qgqBlf-Of5kix-wpuAVmNDtk1tYm4LJyIx-Rvn3SeFbEDGo3bK0c
According to a recent study from the NIH, Novavax, a traditionally created Covid vaccine, does not raise IgG4 levels. It is good to know there is an effective, safe alternative to the mRNA vaccines.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10827267/
It is not totally traditionally. Other concerning news about mRNA vaccine study from Japan.
Increased Age-Adjusted Cancer Mortality After the Third mRNA-Lipid Nanoparticle Vaccine Dose During the COVID-19 Pandemic in Japan
Conclusions
Statistically significant increases in age-adjusted mortality rates of all cancer and some specific types of cancer, namely, ovarian cancer, leukemia, prostate, lip/oral/pharyngeal, pancreatic, and breast cancers, were observed in 2022 after two-thirds of the Japanese population had received the third or later dose of SARS-CoV-2 mRNA-LNP vaccine.
These particularly marked increases in mortality rates of these ERα-sensitive cancers may be attributable to several mechanisms of the mRNA-LNP vaccination,
rather than COVID-19 infection itself or reduced cancer care due to the lockdown.
Researchers have reported that the SARS-CoV-2 mRNA-LNP vaccine may pose the risk of development and progression of cancer.
https://www.cureus.com/articles/196275-increased-age-adjusted-cancer-mortality-after-the-third-mrna-lipid-nanoparticle-vaccine-dose-during-the-covid-19-pandemic-in-japan#!/
A friend of mine/family Dr. in Canada said he has seem some quality of life improvement using Adderal or other similar ADHD meds. Has anyone tried this? I got a few from a friend and I am going to see if that gives me any more energy without PEM issues. I am functional so I am trying to move the needle and possibly be able to do a bit of exercise with weights without having PEM for days after.
Join one of the many CFS fb groups and ask the question. You will get your answer.
We’re have a blog on stimulants coming up. I just started trying them and they are helping….Interestingly they make me calmer. I am having some trouble with sleep, though.
Same with sleep, which one have you tried? I ttied Aderall xr b/c that is what my friend has. I would like to try the one that is not XR but I mentioned it to my family dr and he said I have to get an ADHT diagnosis to them 🙁
Modafinil – I took about a third of a dose yesterday. It did help with my calmness and mental clarity in the morning and I was able to sleep OK.
Re: Stimulants for ME/CFS and a Dr not prescribing them for anything but an ADD/ADHD diagnosis there is this article:
“ME/CFS: A Primer for Clinical Practitioners (2014) “ at the Bateman Home Center site. Check pgs 22, 23. It was written as “Guidelines for Clinical Practitioners” on different stimulants to use and dosages. That being said, look for more current info.
I’M HOPING SOMEONE LESS BRAIN FOGGED THAN ME AT THIS MOMENT COULD FIND MORE UPDATED STUDIES OR INFO. ON “STIMULANTS USE IN/FOR ME/CFS TREATMENT” and post it. I know I’ve seen more.
Stimulants help calm my anxiety, fatigue and get up and get going and then be able to sustain some energy and focus. I have the worst time in the am because my sleep and pain (had)was abysmal for years. Standard dosed Ritalin dampens my pain response SOME. Don’t know if this is common for others. It calms an overly excited nervous system which is why, I believe. It dampens my anxiety, I feel and function more solid and happier than I did before doing so. IT SHOULD ALWAYS BE RX’D UNDER A DRS CARE (my opinion) NOT A MEDICINE TO MESS WITH. This disease can wreak havoc on many systems in the body and neurologically causes chaos. I wouldn’t risk any med without a DR approval and guidance. (not a judgement, just my opinion)
I hope this helps someone. Try to get to a ME/CFS specialized practitioner/Dr or ME/CFS research centers if no other available options in your area. Ask who they recommend nearest your state for a specialist. Just because a Dr sees other patients with ME/CFS or Long Covid does not mean they are well read and knowledgeable on these particular multi-systemic diseases and how to treat them. I learned that the hard way for years. Lot of suffering for my family and I that could have been avoided. WRITE CONGRESS FOR BETTER RESEARCH AND FUNDING (specific info is in above mentioned comments.) Many, many blessings to all.
Cort, can I ask what stimulant you are taking that makes you calmer? It’s interesting because oftentimes I feel coffee can put me back to sleep.
Modafinil 🙂
Hi Cort,
Tonight Rose, whose comments are above, motivated me to watch
Dr. Mel Hopper Koppelman
who talks about …. let’s say…. counter-medical-establishment-and-inaccurate-blood-testing …. and other functional-medicine over mainstream medicine…. in her interview
with Ari Whittenberg
on Youtube.
I would like to point out that Ari has hundreds of podcasts there since he does appreciate emergency medicine and anti-microbial drugs, but sees more wisdom in holistic approaches.
Zoom-out Paradigms.
The only reason I know anything wise (if even I do at all) is that I realized that being too microscopic does not take into account the body SYSTEMS.
Why they work and what impedes them from working.
And we know that Systemic Exertion Intolerance Disease has systemic foundations. Don’t we?
Whether each episode is RIGHT or WRONG does not matter, it is definitely going to be
CHALLENGING of tired concepts in medicine.
But that is secondary.
You gave us a preview that stimulants may help.
They make you calmer.
Through a COMPLETELY different investigative channel, I watched
PhD (but not MD)
ANDREW HUBERMAN
who has his own podcasts
discussed Dopamine in detail.
What we know most recently in ADHD medicine is that
STIMULANTS
make the the brain WAIT for a LARGER stimulation and then release a LARGER DOPAMINE reward. The idea being that too small a dopamine reward is given to the ADHD sufferer.
This helps to conquer the “shiny object” easy distraction for which birds are known.
But humans need to be focused for longer periods on helpful topics in order to be as as successful as we are.
What I had aleady learned in the last, say, 3 years, was that there is not enough dopamine in Fibro to relax the muscles…or… smooth out the response of the muscles. That is, allow them to work…but not to reach MAX and stiffness, a feeling that they may not continue.
I don’t know your story, Cort, not in enough detail. But if memory serves, both CFS and Fibro include fatigue, or non-restorative sleep.
What I would offer, then, is that stimulants helping you are a great start. They may point to the specifics of your subset. And not yours only.
I think, after all that I have read of your GISTs and your fuller analyses,
is NOT AT ALL that you didn’t register what is most important….
Probably you more than anyone did register, step by step,
But, instead, that CFS is not a stereotypical syndrome of symptoms,
each in the same measure for every patient.
In my view, after 4 years, including reading your assessments,
stereotyping subsets may be to microscopic.
You might say “this helped a subset but not every CFS patient”
This does not mean that patients should discount it.
What if they need THAT,
PLUS the next step?
This is SYSTEMIC.
Does that make it STEREOTYPICAL??
or STEREOTYPizeable??
I can’t think of a reason that it could.
HERE IS THE ONLY STUFF THAT YOU CAN AFFORD TO STEREOTYPE:
Inflammation is the 1st step in all disease. It should be brief.
14/15 of the world/s causes of death today are attributed to inflammation,
and the last one is “injury.”
Yes, injury too causes local inflammation. Infection can cause local up to
systemic infection, and systemic infection can lead to systemic inflammation.
My point is simply that :
I think it is not overly profitable to limit a treatment to a subset.
First you would need to detail the subset.
I think it makes MORE sense, useful to MORE people,
to perhaps RATE the effectiveness of various treatments,
and that needs to include a meta-analysis of their symptoms,
and response.
THEN
we could perhaps build a ramp
of minor …. to serious…. to severe CFS and FIBRO
and correlate the effectiveness of treatments to each
stage in the ramp.
It may be that a person has to BEGIN
with one treatment, and if their state is more severe,
then
GRADUATE to the next stage of treatments.
As Always,
I seek FIRST
natural, herbal, nutritional and
physical
things that we can do ourselves with not much money.
If we KNOW what our systems lack
then we can
NOURISH them
Chris
For me, yes stimulants greatly calm my anxiety but only the exact right dose. And they do help with energy and focus. Also, they help me get to sleep and stay asleep. Diagnosed with Adult ADD and PTSD. Have to wonder if it is because of this diseases seemingly relentless attacks on my nerves/nervous system to be honest.
Isn’t that something – stimulants can be calming….Wow.
The use of AI is an encouraging development. Besides the EBV virus, at a minimum its cousin herpes zoster (shingles) seems to be a big problem for people who had COVID or the vaccine. Along with this there seems to be bacterial infections (probably due to immune compromise) and Mycoplasma pneumoniae; probably fungal infections as well. It is concerning that as one pathogen is destroyed, others may take their place. If only we could identify and destroy the trigger of all this effectively.
.
Check out the Model Builder at Work in this blog for an interesting approach to get at more than one virus at a time.
https://www.healthrising.org/blog/2023/06/12/moonshot-ebv-chronic-fatigue-syndrome-inim/
Debbie Moon collected a lot of the gene study information in this article:
https://www.geneticlifehacks.com/genetics-chronic-fatigue-syndrome-and-long-haul-viruses/
I was looking for the FM part from the title. I couldn’t read the chart pictures text, too fuzzy. Did I miss the parts that stated FM?
Article in Australian Journal of General Practice:
https://www1.racgp.org.au/ajgp/2024/april/long-covid-sufferers-can-take-heart
a copy of which has been sent to all general practitioners in Australia. Quote from this article:
“An encouraging step forward is the recent discovery in a preclinical model of a peptide inhibitor of nuclear angiotensin-converting enzyme 2 that reverses the persistent inflammation driving long COVID, reduces the latent viral reservoir in monocytes/macrophages and is associated with reduced SARS-CoV-2 spike protein expression in monocytes from individuals who have recovered from infection.32 It also enhances immune protection against SARS-CoV-2 infection. Clinical trials are pending.”
The study they are referring to:
https://pubmed.ncbi.nlm.nih.gov/37369668/
A Youtube video by these same researchers:
https://www.youtube.com/watch?v=JTYVd9daLAo
Where to buy this peptide:
https://www.probechem.com/products_NACE2i.aspx
I have sent for some and will by trying it by nasal spray.
The Keystone Symposium on Long Covid:
https://nyaspubs.onlinelibrary.wiley.com/doi/10.1111/nyas.15132
This is where the Australians presented their research on the peptide NACE2i.
Another interesting article about another interesting immune system area of study. My only concern with a lot of the suggested treatment trial products that I’m hearing lately, is that IF this illness does end up being an immune dysfunction of some kind then all these symptom treatments will be super helpful and could really do us some good. However if this illness ends up being caused by viral persistence…. Then most of the same drugs could end up being terrible for us! Because they’re often looking at tamping down all these various immune responses, but what if these responses are being caused by a residual virus? Isn’t that going to run the risk of making the underlying illness much worse? Couldn’t we end up feeling significantly better….. For a while, and then bam! Suddenly we’re way sicker than we’ve ever been and the drug appears to ‘stop’ working (probably hasn’t stopped we probably just made these virus worse so we’re sicker).
I feel like we need to proceed with caution here, I’m usually a fan of the argument to be much less cautious when it comes to drug trials – but until we try and get a better grip on answering the main question right now – dysfunction or actively fighting something off – then I feel like we should be very careful indeed. When drugs are being suggested by many different entities right now, in the context of the next step is to test these drugs – I’m not hearing anyone acknowledge this reality – I’m only a lay person, so if there’s some reason NOT to be worried, then I’d sure love to hear about it.
Yes, I’m of the belief that pharma drugs may be the cause of some of these baffling illnesses. I for one took many many rounds of tetracycline years before my immune cascade.Perhaps it’s why a connection can’t be made/found due to years passing by.
What we need to grasp is people in high places have their fingers in the pharma pie.
I for one, and many others with me/cfs can’t take drugs.
Recall folks, the world of “healthcare” revolves around money, greed and profit.
We seen it happen with covid
My alt complement pathway is 227 and i have so much involvement
My pm scl 75 strongly positive
Organ involvement inssussception
Lung disease
Fibrosis
? Encephalitis
Find
I could go on and on and need treatment its been 4 years with no help or treatment
White matter lesions both sides
Phychosis
01639 414135
Thank you, Cort (and Geoff) for another interesting article.
I have a mutation in Complement Component 3 that causes both out-of-control inflammation and defective killing of encapsulated bacteria and fungi. This mutation caused me to have severe post-concussion syndrome after a head injury. I’ve said repeatedly since I got Long-COVID that my hyperactive complement system was part of the reason I was burdened with it (very similar to my concussion experience early on). My particular mutation can be found in 23andMe and Ancestry.com data if you want to check for yourself and is on average in ~1/700 people, though ~1/500 in people with English ancestry (almost none in Asian & African ancestry). You can search 23andMe for this SNP ID (they don’t yet report it): rs147859257 (normal is 2 Ts, my mutation is a T and a G). I can explain how to find it in Ancestry, but you have to download your data. It’s definitively linked to age-related macular degeneration in scientific research, but in our family it’s also correlated with dementia, post-concussion syndrome, early hearing loss, glaucoma, depression, and possibly also early cancer and heart disease. I’m sure there are other innate immune system mutations (e.g., CFH) that also put people at higher risk for both FM and Long-COVID.
Thanks so much for pointing out that mutation! That is a heavy duty mutation. Your story makes me so curious about what DecodeME will find. 🙂
Greetings both Cort and Jennifer.
Jennifer’s information grabbed my attention.
I reread this article from two motivations, and then followed Cort’s link, above, to the 2015 paper which is linked as
(alterations in) Two Complement Genes.
My two motivations to reread this page were:
1. Rob Phair ended his Itaconate Shunt theory interview with the question: but WHY would a cell stay stuck in Innate Immune response ? when Adaptive Immune should take over, creating and antibody, then you are healthy again.
Therefore I read NCBI Bookshelf paper on what the Innate Immune System entails.
2. Cort linked to that complement article and Something in it rang a bell.
1) The Innate system paper mentioned complements and I highly recommend it since it is very much to the point on this innate issue.
https://www.ncbi.nlm.nih.gov/books/NBK459455/#:~:text=Innate%20immunity%20is%20the%20host's,longer%20(days%20to%20weeks).
It explained most importantly that the complement system is responsible for
“coordinating events during inflammation and bridging innate and adaptive immune responses”
Today’s article by Cort mentions those complement upregulations and downregulations.
The ‘Two Complement Genes’ article had this to say about the same two genes being significant in both Macular Degeneration studies and in their 2015 CFS study. And now Jennifer has mentioned the common denominator: nor Macular Degen, itself, but the genes involved.
Incidentally, Macular Degen involves, you could say, a lack of blood vessel tone in the retina, such that the vessels bulge (micro-bulge) if not even bleed (wet mac. degen).
Certainly CFS is the victim of a lack of vessel tone. That’s the whole point: the endothelial cells manage immune response and cytokine release, INCLUDING clotting and thrombosis. That is, unless they are severely reduced in number, or they are dysfunctional.
But center on they “manage immune response”
and therefore it is THEIR complements in question.
When I saw also Shea’ comment further down on having CIRS, i looked it up and it is practically
“Chronic granulomatous disease (CGD) is a genetic disorder in which white blood cells called phagocytes are unable to kill certain types of bacteria and fungi. ”
At least it sounds like Shea’s CIRS would have begun with serious exposure to fungus toxins and bacteria.
And Jennifer I don’t think said her diagnosis was CGD, or I missed it, but they are all closely linked, at least by these 4 descriptions involving genes.
My point is that I didn’t know anything about complements, but now it is clear to how easily their disruption could result in CFS, if it keeps endothelial cells in Innate Immune mode.
It makes some sense to me (understatement) that the genes in question in other studies, and in today’s linked article could be the 1-2% of population reason for developing CFS.
If I did not elswhere mention, while the long-covid international numbers seem wildly higher than American CFS numbers, they too are 1-2 %, but of the world’s population.
The difference between the flus, which in my opinion precipitated rhe majority of CFS, and covid is that covid got lucky as far as its first documented transmissibility, and that if its variants.
What I mean is Covid was found everywhere except Antarctica. I do not think we can say that for each and every flu strain. Or if we can , I don’t think the flu numbers on every continent were as high a proportion as “pandemic” proportion.
I did not expect long covid to have a long haul. But it does. Once I saw the global total of long-covid patients, the reverse occurred (to my brain):
it only worked out to 1-2% of the globe, and J think it is fair (if inaccurate) to say that we all “get” covid in our system sooner or later, but we dont all “contract” it, which means get severely infected.
I do find it bizarre that some people who have been low-symptomatic or non-symptomatic now have long-covid, even some who only received a vaccine (or who THINK they didnt have covid in them).
But that last comment is interesting because
1. CFS looks like this: a, b, f, h i , j, z
symptoms
2. The NIH says long covid looks like CFS (except maybe more organs are CLEARLY affected– as opposed to we don’t know with CFS)
3. People who supposedly had no disease but only vaccine report the same symptoms.
If #3 had covid but it was so mild they weren’t aware, then that leaves only 2 groups.
If #3 definitely did not have covid, then they had what we might call a perverse immune reaction to vaccine.
What it really makes us do is… (now of we include Fibro and MS and others) SEE that this is a human system state, not a flu state nor a covid state. CFS and Fibro have long been said to be idiopathic, or to have no known cause. CFS has been said to be autoimmune but it seems that word has not been used on Fibro. Osteoarthritis is said NOT to be autoimmune, while rheumatoid IS. I believe Osteo IS, and Fibro IS, but for lack of evidence… Then why do rheumatologists treat Osteo???
They all come down to chronic inflammation plus flare-up inflammation. Some: brain inflammation.
While I see solutions as being close-at-hand, I always find gaps in my reasoning and dare not tell you any solution.
But what has come to light in the last decade is: epigenetics:
Epigenetics can mean two things.
1. I had a genetic potential for a problem and a disease switched it ON (DNA *expression)
or
2. I had no gene predisposition, but I damaged my DNA with food or pollution or mould toxins etc
But in both cases, Epigenetics argues that if you can turn it ON, then you can also turn it OFF.
I never thought I would say it, but it seems like disrupted immune complements could be the trigger for CFS and long covid. To be sicker than your peers with flu certainly suggests excessive cytokine action.
I know that the receptors for flus, Sialic Acid receptors, are found in most or all of the same organs as covid’s ACE2 receptors.
–One last point:
my recent reading mentioned NADPH… an antioxidant in the krebs cycle.., could be disrupted. The N stands for Nicotinamide, which is a fancy word for Vit B3.
Guess what Covid does? It occupies the ACE2 receptor meant to accept a converting enzyme, turning the angiotensin 2 vasoconstrictor into a vasodilator. So the infected patient has constant high blood pressure.
When this converting enzyme is blocked by covid, it cascades its effect by NOT triggerring the nicotinic acetylcholine receptor— whose job it is to then increase endothelial cell production of NADPH.
What is supposed to happen during each heartbeat is very quick : tense, relax, repeat. The converting enzymes make this happen.
You might, then, if you read today’s article, plus the NCHI article first page or so, see that “Ah! that’s the same NADPH problem as in covid”
Hi Jennifer Greenhall
So interesting what you said about genes. I looked up my rs147859257 and I have two TT but I have ME and there’s a family history of Macular Degeneration. Also one case of RP Retinitus Pigmentosa. (hopefully spellings ok) Thanks for info.
You could have another mutation in C3 that causes problems or one in a different complement gene or one of many other immune genes. CFH (complement factor H) has several mutations that are fairly common and linked to AMD (one is the inverse of mine in one respect). RP is quite a different disease. Its genetics are less related to immune genes, but it’s good to know your family history. They say RP is the most common genetic eye disease, but I actually think AMD is. The symptoms just occur later with AMD without obvious abnormal development, and researchers are just barely starting to understand all the implicated genes.
Ayurvedic Treatment has been a game-changer for managing my MS symptoms. Since incorporating it into my routine, I’ve experienced reduced pain, increased energy levels, no more blurry vision and a noticeable improvement in my overall well-being I usually get the Ayurveda from natural herbs centre on google search, they guided me through the journey of getting better. I know I’ll get negative comments but I can vouch for this Ayurvedic treatments but you still need to decide what works best for you. Sending prayers
Another great article – thank you, Cort!
My initial diagnosis of MECFS/CIRS in 2019 came from a blood draw of my C4A. The results shouldn’t be over 2,000 something and mine was over 15,000. That was the ah ha moment I desperately waited for after seeing every doctor and having every test throughout 2017 and 2018.
Once I moved into a new home with new furniture, new clothing and a new car, my C4A dropped to 1,450 (normal range).
My symptoms and neuroinflammation improved by 70%.
Prior to removing exposure, I was bedbound with a list of over 27 debilitating symptoms and retractable chronic pain.
Now at 70% improved, the remaining symptoms are PEM, brain fog (much improved but still there), extreme chronic fatigue, chronic systemic inflammation that is tolerable with no treatment whatsoever, and a few others like gut issues, etc.
That said, this article discussing the Complement Immune System hits home for me as that is one of the biomarkers used to diagnosing and treating CIRS.
New research continues to strengthen the possibility that CIRS, MECFS and Long Haulers are all the same condition.
Politics aside, Jordan Peterson recently hosted Dr Richie Shoemaker on his podcast discussing CIRS. Jordan is currently undergoing his treatment protocol after concluding there was enough science to back up his radical claim about a genetic predisposition triggering the body to not produce enough antibodies against foreign pathogens (mostly environmental).
I’m still waiting for the big MECFS break, but remain thankful for people like Shoemaker who dedicate decades of their life to researching this chronic illness.
I’m an ME sufferer who also has many autoimmune diseases. The only things that have ever helped significantly are immunosuppressants (prednisone, Imuran, and Ultomiris). Ultomiris is listed as a candidate in the article.
Ultomiris may have eliminated PEM for me. It’s been less than two months but the effect has been quite remarkable. I’m receiving it because I have a myasthenia gravis diagnosis.