Health Rising’s Sleep Series
- Pt. I – Dr. Bruck Interview – Bronc kicked the series off with an interview of Dr. Bruck – a retired sleep researcher whose son has ME/CFS.
- Pt. II – Ruhoy and Kaufman on Sleep – an overview of a fascinating talk by two long-term ME/CFS/FM and chronic illness experts: Dr. David Kaufman and Dr. Illene Ruhoy on sleep on their Unraveled Patreon podcast.
- Pt. III – A new sleep drug for fibromyalgia? A look at Tomnya – a sleep/pain drug Tonix Pharmaceuticals is submitting for FDA approval.
- Pt. IV – an interview with Dr. Mullington, a long-time sleep researcher and ME/CFS expert who, courtesy of the Open Medicine Foundation, is using cutting-edge technology to further understand sleep in ME/CFS.
Narration
Geoff narrates The GIST
Geoff narrates the blog
There’s Got to Be a Better Way…
People with chronic fatigue syndrome (ME/CFS) can look at fibromyalgia and its three FDA-approved drugs (Lyrica (Pregabalin) – 2007), Cymbalta (Duloxetine) – 2008, and Savella (Milnacipran) – 2009) over 3 years in the US with envy. The truth is, though, these drugs don’t work very well in many people – we’re not missing much.

Tonix is taking a new approach to fibromyalgia (and ME/CFS). Will it succeed where others have failed?
A 2020 review, “Current and Emerging Pharmacotherapy for Fibromyalgia“, stated:
“it must be acknowledged that pharmacological treatment has been met, in general, with rather modest rates of success in this area.”
That was polite researcher-speak for, “It’s amazing how ineffective these drugs are”. A few paragraphs later, they changed that “modest” to “strikingly modest”.
“The strikingly modest progress in this field, as manifested by the surprisingly low compliance of patients,”
THE GIST
- Check out the blog for Geoff’s narration of the GIST and the blog 🙂
- The FDA approved 3 drugs in 3 years (Lyrica (Pregabalin) – 2007), Cymbalta (Duloxetine) – 2008, and Savella (Milnacipran) – 2009) to treat fibromyalgia in the U.S. but studies have shown that most people receive modest benefits from them and don’t stick with them.
- Bringing a drug to market is a tough business. In the past five years or so at least 4 promising drugs (Mirogabalin, Brincidofovir, Synergy, Rituximab) have failed in large-scale studies.
- Tonix’s Tonmya drug – which is heading to the FDA in the second half of this year is an updated version of Flexeril a central nervous system-acting drug that relaxes the muscles.
- Tonmya’s new sublingual format shoots the drug straight into the body, allowing a significant reduction in the dose, and bypassing the toxicity problems that were relegating Flexeril to short-term use.
- Tonix is attempting to kill two birds with one stone. By calming the nervous system down during sleep, it hopes to reduce pain and fatigue, etc.
- Unlike other hypnotic sleep drugs, Tomnya doesn’t help with insomnia. Instead, it produces deeper, more refreshing sleep by reducing the activity of nervous system pathways associated with alertness and vigilance during sleep.
- Tonix seemed like it had a sure bet but its 2019 fibromyalgia trial failed. The company recalibrated and using a higher dose started two large new fibromyalgia trials – only to suspend one after an interim analysis indicated that it was failing as well.
- After an analysis indicated that the pandemic had interfered with the results and after the success of a post-pandemic trial Tonix gave it another go – and launched one last large trial. You can’t say this company doesn’t have faith in its drug!
- That trial was a success. The drug met its primary (reduction in pain) and secondary endpoints (reductions in fatigue, and improvements in sleep) and had an excellent safety profile.
- Tonix believes that with this drug FM patients can have their cake and eat it too. Instead of trading reductions in pain for fatigue (Lyrica) or worse sleep (Cymbalta), they believe that Tonmya will improve both sleep and pain without the side effects seen with these other drugs.
- The company will ask for FDA approval in the second half of this year and expects an answer in the second half of next year. If approval is granted fibromyalgia will have its first new FDA-approved drug in 15 years. Time will tell! Check out a video on the blog for FM researcher Daniel Clauw’s thoughts on the drug.
“Real-life data published over recent years [3], together with the clinical experience of physicians dealing with this group of patients, all indicate that only a minority of fibromyalgia patients continue taking medications for more than a short period of time due to either lack of efficacy, side effects, or both.”
The “strikingly modest” progress may not have been surprising. These weren’t, after all, the most creative drugs in the world: one (Lyrica) was an improved version of Gabapentin while the others were antidepressants (SNRIs). Fibromyalgia and the chronic pain field need a new approach – and in Tonix’s Tomnya drug, they have it.
Getting a drug approved by the FDA is not easy. The examples below will show the numerous and even worse – unanticipated – ways a drug can fail.
Missed Opportunities
Daichii’s Dumpster Fire
Still, drug companies must have been licking their chops at the spate of FDA-approved drugs that occurred in the late 2000s. After 2009, though, the party was over. Despite several attempts, including the notorious Daichii-Sankyo fiasco, no drugs have been FDA-approved for fibromyalgia in the U.S. in 15 years.
Daichii-Sankyo was so confident in their upgraded version of Lyrica called Mirogabalin that it went straight to a huge Phase III trial (3,600 patients in 300 centers worldwide) without doing any Phase II safety and efficacy trials.
It was easy to understand their confidence. Mirogabalin was supposed to be Lyrica without the side effects, but the drug utterly bombed. In two unusual reports, the company argued both before and after the trial that the new outcome measures the FDA had put in place for FM made it more difficult for drugs to be approved.
*See “Did the FDA Mess Up the Biggest Fibromyalgia Trial Ever?”
Bye-bye Brindcifovir
Some people may remember CMX-001 or Brindcifovir – the next great antiherpesvirus drug. Studies suggested that this Vistide upgrade was effective against many types of viruses, leading the FDA to give it fast-track status for no less than three separate viruses.
Brindcifovir sailed through large Phase 2 trials but then stunk it up in its Phase 3 trial (perhaps because of something the doctors unwittingly did). Subsequently, Chimerix, its manufacturer, halted two more phase 3 trials and ultimately sold the drug to another company.
Sorry Synergy
Over in ME/CFS, the phase II Synergy trial combining methylphenidate and mitochondrial nutrients seemed to be a lock before it, too, failed to meet its primary endpoint. The unusually high placebo effects may have doomed it. (It did well in a Gulf War Illness study.)
The Big Hurt – Rituximab Bombs
The one that really hurt, though, was Rituximab which aced early trials only to bomb in its Phase III trial for ME/CFXS. (One ME/CFS researcher believes a reduced maintenance dose may have been the culprit.)
There are clearly many ways for a drug – even potentially a good drug – to fail.
A Better Way?
Health Rising has been following Tonix Pharmaceutical’s Tonmya (formerly TNX-102) story with fascination and, at times, horror for about five years. Tonix has been anything but exempt from the pitfalls that drug companies can experience. The notable thing about this small drug company has been its persistence. It’s been like the little engine that could: through thick and thin it’s just kept pulling this drug up the mountain.
An updated form of Flexeril (cyclobenzaprine), TNX-102, or Tonmya charted a new path. Tonmya is a muscle relaxant that relaxes muscles via the central nervous system. Its new sublingual format shoots the drug straight into the body, allowing a significant reduction in the dose, and bypassing the toxicity problems that were relegating Flexeril to short-term use. By reducing the activity of nervous system pathways associated with alertness and vigilance during sleep – and thereby relieving pain as well, Tonix aimed Tonmya at key areas of FM and ME/CFS.
It seemed that more refreshing sleep AND reduced pain might be on their way for FM patients, but the 2019 trial failed. Its interest in FM apparently over, Tonix reported that it would try again with PTSD. When its PTSD trial trial succeeded with a larger dose, though, Tonix thought it had its answer.
Reinvigorated, the company started two new, large, Phase III FM trials (RELIEF, RALLY) using the higher dose. After an interim analysis suggested the drug would not make its primary endpoints, Tonix abruptly suspended the RALLY mid-trial. Tonmya was, once again, on the rocks.
Further analyses suggested that Tonix had found a new way to fail a treatment trial. While it’s analyses suggested the RALLY trial successfully reduced pain levels, the high dropout rates occurring during the pandemic on both arms of the trial (TNX-12, placebo) scotched the kind of analysis the FDA required.
Tonix’s successful RELIEF trial, which began after the pandemic, convinced Tonix that despite two expensive trial failures, it was on the right track. The little drug company that seemed never willing to say no started its aptly named RESILIENT phase III trial. No one can say Tonix hasn’t had faith in its drug.
Resilient Indeed
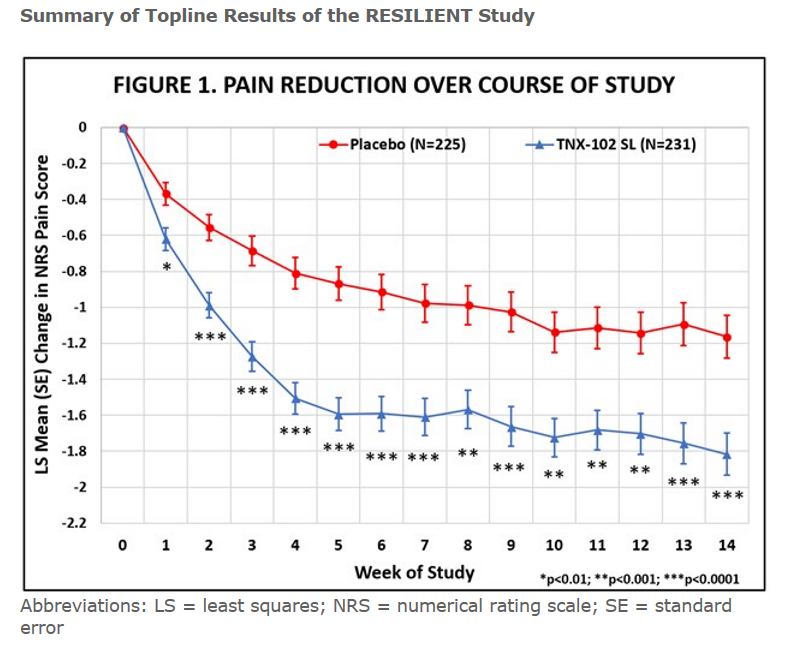
The results of the 457-person trial are in. Tonix reported that this time the drug met its pre-specified primary endpoint by significantly reducing daily pain compared to placebo (p=0.00005). Tonix reported that clinically meaningful results were also seen in all major secondary endpoints related to improving sleep quality, reducing fatigue, and improving overall fibromyalgia symptoms and function. Almost 50% of FM patients taking the drug reported at least a 30% decline in pain. The drug worked quickly as well and started to have an effect in the first week of use. Its effect size of .38 is considered a “moderate” effect size.
Abbreviations: LS = least squares; NRS = numerical rating scale; SE = standard error.
Daniel J. Clauw, M.D., a well-known fibromyalgia researcher and Director of the Chronic Pain and Fatigue Research Center at the University of Michigan, called the results “terrific news for patients with fibromyalgia”. He noted that the drug’s active ingredient has a “known, favorable safety profile from decades of use” and that it was beneficial “in many other key symptom domains, including sleep quality, sleep disturbance, and fatigue”. (See Dr. Clauw and another long-time FM researcher Leslie Arnold in the below webinar.)
Tomnya also appears to be quite safe, with the most common systemic side effects – headache and somnolence – occurring in about 3% of patients. No weight gain, as occurs with Lyrica or antidepressants, was found.
Indeed, Tonix believes its drug is unique in fibromyalgia pharmacology because it doesn’t present any tradeoffs. People who take Lyrica to reduce their pain often have less energy. Likewise, people who take Cymbala or Savella often experience worse sleep. They believe Tonmya can improve pain, sleep, and fatigue.
The Sleep Angle
For Tonix, sleep is where it’s at. Seth Lederman, Tonix’s president referred me to Dr. Iredell Iglehart III’s 2003 patent which described a drug that reduced pain in fibromyalgia (as well as ME/CFS) by improving sleep. The novel part of Iglehart’s patent was that a low dose of cyclobenzaprine (or a metabolite of cyclobenzaprine) was required.
The goal for Tonix is not to produce longer sleep nor to prevent insomnia but to produce deeper, more refreshing sleep. It believes that non-restorative sleep may be the Achilles heel of FM and ME/CFS. Moldovsky’s pioneering Toronto studies in the 90s, and others since then, have indicated that poor sleep increases pain sensitivity.
In 1993, Moldovsky reported that “chronic fatigue syndrome and fibromyalgia have similar disordered sleep physiology, namely an alpha rhythm disturbance (7.5-11 Hz) in the electroencephalogram (EEG) within non-rapid eye movement (NREM) sleep that accompanies increased nocturnal vigilance and light, unrefreshing sleep.” It’s the “nocturnal vigilance” and light, unrefreshing sleep that sets diseases like ME/CFS and FM apart. More recent research calls this a “hyperarousal sleep disorder”.
Tonmya is different from other sleep drugs in that it blocks 4 receptors associated with increased alertness. It’s notable that the hypnotic sleep drug Ambien, which does increase sleep times but does not affect deep sleep, did not improve pain levels in FM.
Tonix proposes that the brains of FM and ME/CFS patients are simply too alert. Indeed, several studies suggest that the sympathetic nervous system (fight/flight) activation and sleep fragmentation play a key role in these diseases. It may not be so much that ME/CFS/FM patients are not getting enough deep sleep but that their deep sleep is not deep enough. A 2020 Australian ME/CFS sleep study concluded:
“Autonomic hypervigilance during the deeper, recuperative stages of sleep is associated with poor quality sleep and self-reported wellbeing.”
In short, ME/CFS/FM in several ways, seems tailor-made for a drug that can help the brain to calm down and rest during sleep. That’s what Xyrem (sodium oxybate) – the sleep drug that came close to being approved by the FDA for sleep – does. Xyrem was rejected, not because it didn’t work in FM, but because of its use as a date rape drug.
Timeline
Tonix plans to submit a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) in the second half of 2024 for Tonmya. Lederman told me it’s taken 700 hundred million dollars (!) in funding to get this far, but Tonix and we should know by the second half of next year if fibromyalgia finally has a new FDA-approved drug. If Tonmya is approved, it could hit the market 3 months later. Time will tell!






Oh my, this is exciting news. I pray this small company finds the funds to continue with its unrelenting patience and progress.
My adult daughter with cognitive and physical injuries from a significant car accident 30 years ago now has tried everything – including Botox injections – to no avail. She has been so patience waiting for relief from pain and sleep disorders – she deserves a medal.
As a single, senior now – I will try to scrape up a donation towards this new promising drug.
Thank you for this article.
God bless ,
Re:medal…..Maybe its my warped way of thinking but, i keep thinking my city should be naming a new road after me,for all the crap we’ve all been through…. that’s how sick and ignored i am/was. I still cannot believe a person can be this sick and totally ignored.
You are not alone. Your comment helped me feel less alone. Thank you. I’m sorry for your healthcare troubles. May God Bless us all.
Hi Cort,
Getting this information about a possible medication has made my day & renewed hope that I, at 86 may still have time to get relief from a lifetime of FMCFS. I tried the three medication that were approved by the FDA when they were available, but they didn’t work.
Today my mind is very foggy & I’m overwhelmed with this life in general. I read the Gist’s that you write as I don’t understand all the medical information & I will listen to Daniel Clauwes video that you included in your Gist. My faith keeps me going & Cort, you are included in my faith. I pray that this medication will be our saving grace! Thanks for everything that you do, even while dealing with your own illness, that I think people tend to forget.
Kay, I so resonate with everything you said. Older myself now, and have had CFS/Fibromyalgia for decades. Lack of Sleep has become such a darn difficulty now in my life. This is all very promising and gives , again, hope . Maybe not a cure, but at this point in my life, I no longer care. I would just like to get sound,consistent sleep, without drs looking at me like it’s my fault. And what you said about Cort is absolutely right on!
Hi Jeanie, I’m embarrassed, but with my foggy pain I forgot to answer you. Along with FMCFS I have found out that getting older causes more problems. I inherited hypothyroidism from my Mom & her Mom causing an under active thyroid that has a bearing on most of my problem including FMCFS. I’m suffering with my edema (big time) & I have found it gets worse with age.
I have sleep apnea & for years wore a mask, but gave up because taking the mask on & off to go to the bathroom kept me awake. Now like you, I go to bed tired & get up tired, & after years of trying to keep a schedule, I’ve given up. I’ve gone from a morning person to being awake all night until I take my pills in the morning. I am also excerise intolerant. I’m praying that they will find something to help all of us, especially the young ones so they can live a normal life. My doctor & I have agreed that I have become so sensitive to medication that trying new or old ones I have tried, just creates more side effects that I can’t handle. Now I’m just using the medications that I have been taking for years.
Cort, thanks a million & keep up the good work.
Look into whether you have the MTHFR gene mutation. It is highly correlated with autoimmune disease and Fibro. You may need to take methylfolate. Yes, Tonmya will be a welcome reprieve.
Want revenge on Fibromyalgia? Buy TNXP stock. Tonix Pharmaceutical stock is at an all-time low. (TNXP at .61 cents today.) Tonix shelled out $74 million to get this drug to market, so the company finances look bad for now. But this drug will at least dominate the Fibro market at launch, worth $2.9 billion now and $4 billion in 10 years. The global pain management market is $78 billion and will climb over $90 billion in 10 years. Add in Chronic Fatigue market, IBS, Insomnia, and all the others mentioned in the article…Tonmya will dominate it all as first-line treatment due to its low side effects, extremely high patient adherence, safe history, and non-addictive qualities, in contrast to opiates. It’s a market disrupter that will be prescribed as routine standard practice. Profits from Tonmya will anchor the other R&D projects at Tonix for the drug’s patent duration of 15-20 years. Little Tonix will become a monster. Look up the story of OxyContin, Purdue Pharma, Sackler family: $35 billion profit. That is the scale the drug will have: $35 billion profit, but safe to use.
The CEO has been diluting shares with new stock issuance in order to bring Tonmya to market. As long as the stock doesn’t drop low enough to be pulled off the stock market listings, this stock will shoot sky high.
Anyone know if/when Tonix will come to the uk?
After three and a half decades of dealing with FM, this new drug sounds very promising. Knowing how drug companies operate, most of us may not be able to afford the high price this drug may command, assuming it becomes available. For those of us on Medicare, it may take years for it to be approved. For profit health care always puts profit ahead of people. Interestingly, the new weight loss drugs received a very quick green light from Medicare. We may have to organize and complain to let politicians know we are tired of being ignored, abandoned and called mental cases. Sick people don’t have a lot of energy to fight political battles.
Thank you for bringing up Medicare. I need to keep my hope in check timewise.
So true! 2 decades here!
With any new drug what will be the cost and will it be covered by insurance/ Medicare etc.
I don’t know. I will try to find out. These drugs are tremendously expensive to bring to market so I imagine it will not be cheap and having it covered by insurance will be key.
As with any new drugs they will be patented with the company until they are released as a generic. The cost will be enormous and mostly either not covered with the insurance formulary. Sadly profit before patient care.
I’ve kind of changed my tune on this after reading “The End of Cancer” where DeVita goes to town on the FDA. He cites numerous things the FDA that makes it really hard to bring drugs to market or get them to patients – even dying patients.
Ledgerman told me it’s taken $700 million to get the drug this far. That’s just crazy. The investors have to see returns or they’ll never invest that kind of money. We have to find a way to make drug development less expensive.
My hope is that the market will be so large that Tonix will keep the price down in order to get it to more people. I’ve been diagnosed with both ME/CFS and FM as I imagine that many people have. Or people with ME/CFS that fit an FM diagnosis could make sure that they have one in their medical records. I would get that in there now…
I doubt it took 700 million Cort.Corporations juggle numbers and lie big time….all the time.
I quit being gullible
Years ago
That may be so – it seems awfully high! But check this out
How Much Does It Cost to Research and Develop a New Drug? A Systematic Review and Assessment
Estimates of total average capitalized pre-launch R&D costs varied widely, ranging from $161 million to $4.54 billion (2019 US$).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8516790/
It’s incredibly expensive.
No offense Cort but…I’ve been around the block long enough to know that anytime money, profit and shareholders are involved,the skies the limit on how far they will bend things to hear that
Chinga changa in their pockets.
The last conglomerate I would believe is the fda
Throw in the fact that people like us that are desperate and very vulnerable searching for help for decades will go to many lengths and try many drugs to find relief.
I’ve always been of the belief that most of us can’t tolerate drugs🤷♂️…I know I cannot
I quick summary of my long story tells that I had to sue a large insurance company to get my dis.
The entire process leading up to pretrial was a joke.
I had to turn my lawyer into the law society. He later took people funds held in trust and liquidated at the nearest casino.
Then the law society gave him a hand slap and offered his license back.He declined as his reputation is shredded in these parts
I could write a book about what happened to me
You see, it’s about the money$$
Here in CAN🇨🇦 CAN land it takes sometimes decades just to get a diagnosis and it’s all for one reason…they want you to get onto the big wheel and go from Dr. To dr. To dr. So they can all profit as they get paid…billed directly to the govt.
meanwhile the patient is in disbelief that they’ve been to 30, 40, 60…sometimes 100 apointments with still no diognosis.
The system up here is truly corrupt.
I am a very inquisitive person by nature so I was/am always looking at the angles.
I’ve learned where there’s money involved,greed and corruption always rear it’s ugly head.
Sorry for sounding so negative as most of my posts seem but I’m 40 years in and am of the belief drugs did this to many of us in the first place.
Nobody knows what these drugs do long term.
It took years for the damage that tetracycline caused to me to show up.
Sorry you’ve had such a bad time but I don’t believe its the Canadian medical system. My doc diagnosed me in 1996 at the first appointment I had with the sudden onset of bewildering symptoms. I had never heard of Fibromyalgia so went home and hopped on the computer. I was happy to discover it was all in my head as that sounded more hopeful than what my doc said. However, after many tests and appointments with many specialists who all confirmed my GP’s diagnosis I really had no choice but to believe it wasn’t in my head and it wasn’t going anywhere. I have heard of folks who had trouble getting diagnosed but I know people like me who didn’t. I never had trouble getting short or long term disability (several times over the years) right up until I qualified for CPPD. So I don’t believe it is a “corrupt system”.
Sandra, need I remind you that the first 30 or so years of this, many of us have been told we were crazy…totally ignored and/or passed off as lazy.
Need I remind you that all these names fibro,me, cfs,long covid, etc. Are all one of the same.
Ever wonder why all these made up names keep changing?
Ever wonder why the medical “industry” never,ever cures any person of any illness,yet people on their own,cure their own illnesses…then when they try to tell the medical experts how they cured themselves…it’s met with skepticism.
A person cures their cancer with ivermectin ($12)but oncologists deny it happens…Ever wonder why.
No, you need not.
I know little about stocks, but my husband has invested in some biotech companies. He bought Amarin and Novavax. Both are small companies with great ideas that have faced challenges with FDA approval while the big companies like Pfizer sail through the approval process. The smaller companies seem to have a much harder time and seem to endure more scrutiny in my opinion. According to my husband Tonix is in dire need of funding. It is going to need an infusion of cash in order to bring this drug to market.
Also, some of these small companies suffer from mismanagement on the business end. Even when they get approval, they do not have the marketing budget to get enough attention.
I agree with you here, Cort. If any of you qualify for a FM-diagnosis (or ME/CFS having only an FM-diagnosis now), try to get diagnosed with both. That way, if a medicine becomes approved for FM or for ME/CFS, you will have a better argument for getting to try it out.
It is so difficult to get medications that may work, do whatever you can to have more legs to stand on.
I have both diagnoses, I am hoping this will help me when I get a visit at a pain clinic this year.
Also, commenter Nugget above can definitively be right: this can potentially become a HUGE stock-option. If you have ever considered investing in stocks, this has the potential to take off, with huge profits.
But do not take my word for it, do your own research. I am researching it more now aswell. And you will have to keep the stocks to atleast 2025, or longer. So think long-term investment.
Interesting. I found that Chinese skullcap does this for me. My deep and REM go from about half the normal % lowest range to slightly above the lowest range within the median. I also take magnesium and l-theanine with it, but they don’t work alone.
Hi Bob, How do you measure your sleep? Thanks
I use Fitbit. It’s probably not the most scientific but it seems pretty consistent with how I feel when waking.
This is very interesting.
I remember telling my husband and then my primary caregiver if there was a medication that I could take that would help me to relax during my sleep, I felt ot would help me.
Unfortunately, I was told that there was nothing and sleeping pills tend to become habit forming.
I often wake up in quite a bit of pain.
I often notice myself clenching my jaw, neck and shoulders.
I even started wearing a timer that would alarm at any interval from 1 minute and up.
When it would alarm, I would check my muscle tension and reset them to a more relaxed state.
This did help but then I would become so used to the alarm that I would not notice it going off.
When things get so tight, and I feel like my muscles are rock hard, I will pull the alarm out and use it.
I also have quite a bit of difficulty sleeping when I have a lot of stressors, especially ones with time lines, going on in my life.
I don’t understand why, but magnesium body butter can give me great temporary relief.
I have dysautonomia so it is interesting tgat was mentioned in this article!
I sure hope this drug gets approved.
I’m not one to trust the pharmaceutical companies, but this one seems to really care, not just to make billions of dollars.
Thank you to the people that have persisted in showing that something can help fibro patients get significant relief with this drug.
It’s that kind of arousal that makes me excited about this drug. It might fit you really well with all that muscle tension you experience. Flexeril, the drug Tonmya was created out of, started out as Flexeril – a muscle relaxant. 🙂
Cort, I posted about flexeril but don’t see it. The interesting thing is flexeril is similar in composition to the TCA drugs like elavil. They are now being used by gastro docs for irritable bowel syndrome at lower doses than used for depression…around 10-25 mg. elavil is an old drug and has many side effects as all drugs do but it has some excellent qualities like sleep, help with pain and discomfort of IBS, depression, and nerve pain. It does have a drying effect like dry mouth and eyes. It is anticholinergic. So, some may want to get flexeril but not easy to do for some so they might want to consider Elavil or Pamalor.
Please let this be the one to help us.
I am crossing my fingers so hard that FDA approves it. The data are solid so I would think they should approve it.
Let’s hope. The only question in my mind is effect size – .38 moderate
Terrific! I took Lyrica but very quickly I had vertigo. That’s really scary and I stopped taking it. Interestingly Tonix’s stock (TNXP) price has been down 55% the past 30 days and down 64% year to day.
It seems really strange and makes me wonder what’s going on. The company recently sold new stock shares in order to raise capital to carry it through, I guess, and its stock dipped. It was down even before that though.
They are in bad financial shape. I hope they can figure it out. According to my husband that follows stocks, the price has fallen so low, they are trying to do a “reverse split,” which, as I understand it, is a two for one split in order to stay listed on the stock exchange.
They did a 32 to 1 reverse split on June 6! It also announced more shares offering today and the share price crashed by 45% afterwards!
Is there a link to the video of Dan Clauw on the new drug? Thanks a lot!
I haven’t seen the video but I was told Clauw is in it. It should show up in the middle of the blog somewhere.
I would be very curious to know how this drug might interact with either central or obstructive sleep apnea. I have an apnea that comes and goes, and therefore is difficult to detect with the typical overnight sleep study. It’s horrible to wake up several times per night in a deoxygenated state (with plus oximeter readings of 81%) and no doubt this has conditioned me to sleep even more lightly than my ME/CFS already causes. It’s also meant that I’m much more wary of sleep-assisting drugs. However it seems that H1-blocker ‘Loratadine’ and mast cell stabilizer ‘Quercetin’ seem to ameliorate the problem.
Tonmya sounds amazing, the way it might address the issue of poor sleep which perpetuates our conditions. But can anyone point me in the right direction to learn how it or similar sleep deepening drugs might either worsen or alleviate sleep apnea?
I have this problem, too, Geoff. I have found that the H1 blocker “desloratadine” works better than plain “loratadine”. You can get it cheaply as a generic.
Quercetin also works, but you have to use some kind of modified quercetin to boost absorption. You can get “bioactive quercetin EMIQ” which means “enzymatically modified isoquercetin” made by Natural Factors. It’s relatively cheap and available everywhere. For this to work you need to take at least 2 with each meal. I take 3 of these four times a day and I don’t have the apnea anymore.
That’s wonderful Ann1 thanks so much for responding with such helpful information, from the authority of lived experience. I will look into that for sure and ask my Dr about a prescription.
Geoff you don’t need a prescription for either of these!
Desloratadine and Bioactive Quercetin EMIQ by Natural Factors are available at drug stores and health food stores, respectively. Go take a look!
According to the Mayo clinic website it says prescription only for Deslortadine: https://www.mayoclinic.org/drugs-supplements/desloratadine-oral-route/side-effects/drg-20067466?p=1#:~:text=This%20medicine%20is%20available%20only,possibly%20life%2Dthreatening%20side%20effects%20.
…but I’m in Canada so I’ll check if it’s OTC here or needs a prescription. I’d prefer a prescription as it would be covered and I wouldn’t have top seen my grocery money on being able to breath at night LOL. As for quercetin I already take it with bromelain which increases bioavailability. But I started taking it at night as well as morning and I think it helps. Again thanks for your response because it does seem that this apnea may be mast cell mediated so it’s good to have that theory validated by the experience of others. Cheers!
Geoff, I live in BC and desloratadine isn’t prescription, and I’m not sure they would give you a prescription for it because of that.
As for quercetin, the kind with bromelain is ok, but not great. You’d have to take a lot of that to feel any effect. The enzymatically modified isoquercetin is much better. Natural Factors sells it for $25 but you can get it cheaper online. I never pay more than $18 for a bottle of 60.
Geoff, Costco sells Kirkland brand bottles of desloratadine OTC at the pharmacy. They also sell Aerius (which is desloratadine), but it’s more expensive.
Both at Costco have more in the package than other stores.
Hi Ann1,
A question about your response to Geoff, please:
‘For this to work you need to take at least 2 with each meal. I take 3 of these four times a day and I don’t have the apnea anymore.’
Would you please clarify what you take 2 of with each meal, and what you take 3 of four times a day?
And, how did you discover this combo and dosages? I’d like to learn more about how these help with sleep apnea as it may be useful to myself and my father.
Thank you!
Tracy, I’m taking about quercetin, specificaly enzymaticaly modified isoquercetin (EMIQ), 50mg. This works for me because I have MCAS. It won’t work for someone who only has regular sleep apnea.
Thanks for the recommendation for desloratadine at Costco. I will check it out.
It’s my firm belief this claim of focusing on sleep medications that help suppress and/or calm the CNS is on point.
As an MECFS sufferer for 8 years, taking benzodiazepines every night for sleep has given me a marked improvement in overall symptoms and quality of life — specifically better sleep, reduced pain and more energy. No question about it.
Good to hear – and thanks for sharing your experience. 🙂
Shea, may I ask if you feel less effect of benzodiazepines over time using it every day?
I use benzos at times to sleep or to help me balanse my bipolar disorder. It has greatly helped me with bipolar disorder (I am now episode-free for years after I was allowed to use Valium whenever I needed to, aswell as using blue-light blocking glasses actively).
I also feel it has helped my sleep at times an ME/CFS, but I’ve been afraid to use it every day. I am interested in your experiences as a long time user.
Thanks for replying if you get the energy and time!
Thanks for this article, really hopeful. Most definitely lack of or not deep enough deep sleep is a culprit in me/cfs & FM. The audios are excellent & helpful by the way. Thanks for including them.
Unfortunately, Tonix is not a well managed company. I did some research, and Tonix has the honor of being the biotech currently most rapidly heading toward bankruptcy.
Thanks for another great post! I am curious about the effect on restless leg syndrome.
I have fibromyalgia been on many meds i currently take celebrex it helps some and mucinex helps removes uric acids inflammation also the ozempic pen.
Do need to get of Pregablin as does not seem to work but dissociate. So hope they get approval
É bastante promissor e já me sinto aliviada com essa notícia! Por outro lado, temo pela nossa saúde por outro aspecto. Já vou me explicar: assisti à série “O Império da Dor” (eventos reais), e vi o quanto há de manipulação de forma geral para a aprovação de um medicamento. Um membro da FDA (muito honesto e ético) acabou sendo corrompido para aprovar um analgésico (opióide) para tratamento de dor crônica (Oxycotin/Oxicodona), comercializado pela Purdue Pharma e cuja composição química levava heroína. Por esse motivo, causava grande dependência e quem tentava se . A posologia precisava ser aumentada a cada ida do paciente ao médico (muitos profissionais também corrompidos), até que se tornava uma situação insustentável. Em busca de alívio da dor crônica, pacientes compravam o remédio nas esquinas, através de traficantes. Farmácias eram assaltadas. Famílias foram destruídas e vidas se foram por overdose.
Depois que assisti a essa série, sinceramente, fiquei muito desconfiada com relação às minhas necessidades medicamentosas. Sofro de fibromialgia, depressão e ansiedade (uma coisa leva à outra!) e agora, desconfiada, tenho medo de qualquer substância que esteja em fase de teste e aguardando aprovação.
Google Translation:
It’s quite promising and I’m relieved with this news! On the other hand, I’m afraid of our health for another aspect. I’ll explain: I attended the series “O Império da Dor” (real events), and I saw how much manipulation was done in a general way for the approval of a drug. A member of the FDA (very honest and ethical) ended up being corrupted to approve an analgesic (opium) for the treatment of chronic pain (Oxycotin/Oxycodone), marketed by Purdue Pharma and whose chemical composition was heroin. For this reason, it caused great dependence and was tempted to be burned. The dosage needed to be increased with each patient going to the doctor (many professionals were also corrupted), so that it became an unsustainable situation. In search of chronic alimony, patients bought or remedied on street corners, through traffickers. Pharmacies were assaulted. Families were destroyed and lives were formed by overdose. After I attended this series, sincerely, I was very suspicious