

Check out Geoff’s Narrations
The GIST
The Blog
THE GIST
- Could people with diseases like long COVID and chronic fatigue syndrome (ME/CFS) be aging more rapidly than normal? Recently, a large review paper made that case in long COVID (and referred to ME/CFS).
- The authors demonstrated that processes associated with aging such as chronic inflammation, immunosenescence (exhausted T-cells), dysbiosis, mitochondrial dysfunction, cellular senescence, and metabolic dysregulation have all shown up in long COVID (as well as ME/CFS). Similar problems have shown up over time in people with exposed to another infectious stressor – people with HIV.
- The first anti-aging treatments they focused on were involved in, what else, on tamping down chronic inflammation – likely a key issue in long COVID, ME/CFS, and many chronic diseases. They proposed a variety of treatments (etanercept, prostaglandin EP2 receptor antagonism agents, and NLRP3 inflammasome inhibitors (MCC950 (also known as CRID3 or CP-456,773), OLT1177 (Dapansutrile), CY-09, Inflamazome’s compounds.) some of which are being trialed in long COVID.
- With regard to diet, they focused on reductions in butyrate producing bacteria in the gut. Reductions of these gut protective bacteria have been linked to literally dozens of chronic illnesses and a breakthrough in finding ways to repopulate the gut with these crucial bacteria (or to increase gut butyrate levels) would be beneficial indeed.
- With regard to mitochondrial rejuvenation, the authors pointed to NAD+ and/or its precursors to combat NAD+ declines seen in aging. They reported that NAD+ declines are “a salient feature” of ME/CFS. They also proposed trying out urolithin-A – a metabolite that’s been shown to improve mitochondria functioning, clean out old and inflamed mitochondria, reduce neuroinflammation, reverse cognitive decline, improve muscle functioning, and prolong life spans in laboratory animals.
- They pointed to a class of drugs we haven’t heard of before – senotherapeutic or senolytic drugs that clean out old, moldering cells that just won’t die and are pumping out inflammatory factors. Getting rid of these cells is a main focus of the longevity movement and the authors illuminated dozens of potential treatments including hyperbaric oxygen treatments that appear to be able to do this.
- In the last treatment section they focused on metformin and Rapamycin – which have been able to increase lifespans in animals, reduce inflammation, and increase gut diversity (metformin), help with T-cell exhaustion (Rapamycin), restore autophagy (both) and others.
- The authors ended by describing long COVID as a disease in which homeostasis – the ability to maintain biological stability – is under attack. The authors refer to this ability to snap back as resilience or “physiological reserve”, which wanes as we age. Simply put, the body lacks the ability to recover completely from, in the case of long COVID and many people with ME/CFS, an infection. ME/CFS has been described in a similar way numerous times over the years.
- While it’s a bit wrenching to think that, on top of all the other stuff going on in these diseases, one might also be biologically aging more rapidly, the good news in all this is that the longevity, or geroscience, field is booming with billions of dollars of research funding going into it every year.
- If diseases like ME/CFS and long COVID are causing accelerated aging people with these diseases might very well benefit from the interest in aging research and the new treatment options that are sure to follow.
Recently, a large review paper made the case that the biological process of aging has accelerated in long COVID. The paper, “Long COVID as a Disease of Accelerated Biological Aging: An Opportunity to Translate Geroscience Interventions Accelerated Biological Aging in Long COVID“, was produced by an interesting blend of Saudi Arabian, Egyptian, and U.S. researchers from the Mayo Clinic and Northwestern University.
A New Focus?
Epigenetic modifications build up over time. Could the epigenetic clocks in ME/CFS and long COVID be sped up?
The authors asserted that long-COVID researchers should focus on fundamental processes associated with aging that have shown up in long COVID such as chronic inflammation/immunosenescence, dysbiosis, mitochondrial dysfunction, cellular senescence, and metabolic dysregulation. (How interesting that both ME/CFS and long-COVID research is already naturally trending in many of these directions.)
Interestingly, the first instance of accelerated aging the authors pointed to was a post-viral one: HIV. About 15 years ago, HIV researchers began picking up signs that HIV survivors were aging more rapidly than normal. This showed up in an increased incidence of disorders associated with aging, and biological evidence.
Their epigenetic “clocks”, for instance, appeared to be sped up. (Epigenetics refers to changes that alter the expression of our genes over time. The longer we are alive, the more epigenetic changes that occur.) They also exhibit increased markers of innate immune-driven inflammation and immune dysregulation linked to aging. Increased levels of exhausted T-cells, problems with natural killer cells, dysbiosis (gut flora dysregulation), mitochondrial dysfunction – all of which have been found in ME/CFS – and are also associated with aging – have shown up prematurely in HIV patients.
Referring at times to ME/CFS studies, authors spent over 20 pages detailing what they believe are signs of increased biological aging in long COVID. They included:
increased levels of exhausted CD8+ T cells, latent herpesvirus reactivation, a hyper-immune response to those viruses (leading to T-cell exhaustion), dysregulation of B-cells, elevations of the chemokine eotaxin (CCL11), microglial activation (neuroinflammation), brain and neuronal injury (increased tau proteins and neurofilament light chain (NFL)), gut microbiome dysregulation, leaky gut, mitochondrial dysfunction including impaired fatty acid metabolism (perhaps produced by herpesvirus activation), a shift toward anaerobic metabolism, DNA damage, reduced telomere length, and others
Potential Treatments
The authors of the paper outlined several treatment areas they believe show promise in turning back the clock, so to speak.
Anti-Inflammatory Agents
The first anti-aging treatments focused, what else, on tamping down chronic inflammation – likely a key issue in long COVID, ME/CFS, and many chronic diseases.
They proposed assessing treatments used to treat cardiovascular diseases (canakinumab and colchicine) and anti-inflammatory treatments that have been shown to extend lifespan in mice such as etanercept, prostaglandin EP2 receptor antagonism agents, and NLRP3 inflammasome inhibitors (MCC950 (also known as CRID3 or CP-456,773), OLT1177 (Dapansutrile), CY-09, Inflamazome’s compounds.
The good news is that inflammation has been identified as potentially a key mover in long COVID and many anti-inflammatory clinical trials (dexamethasone, plasma exchange, plasmapheresis, intravenous immunoglobulin (IVIG), RSLV-132, baricitinib, anakinra (IL-1β receptor antagonist), sirolimus, deupirfenidone, complement pathway inhibitors (ROCUNEST, Zilucoplan are currently underway in long COVID)).
Diet
Given the link between gut flora (microbiome) problems and inflammation, it was no surprise to see dietary manipulations make the list of possible anti-aging therapies. The gut is often given short shrift in these diseases, but the evidence of its importance to chronic diseases continues to pile up.
A huge 2024 type 2 diabetes gut study, which involved 10 prediabetes and diabetes cohorts in three countries (yes, diabetes has a lot of funding!), concluded that changes in the gut came first and set the stage for type 2 diabetes. The lead author stated, “Therefore, we are confident that the observed changes in the gut microbiome happen first and that diabetes develops later, not the other way around.”
The reduction of butyrate-producing bacteria in type II diabetes could not have been a surprise given the similar reductions found in other diseases including (take a seat…) Gulf War Illness (GWI), fibromyalgia, and ME/CFS, inflammatory bowel disease (IBD), Crohn’s disease, ulcerative colitis, irritable bowel syndrome (IBS), colorectal cancer, hypertension, metabolic syndrome, Type I and II diabetes, multiple sclerosis, rheumatoid arthritis, Sjogren’s Syndrome, systemic lupus erythematosus, atherosclerosis, stroke, Parkinson’s disease, Alzheimer’s disease, and others.
Eventually, I gave up – every chronic disease I looked at featured reduced levels of butyrate producing bacteria. Butyrate reductions are such a common element of chronic illness that one must assume that efforts are underway to find ways to boost them. Dozens of clinical trials are underway.
The authors noted that the gut is receiving its due in long COVID and that probiotics, larazotide acetate (a gut permeability regulator), and fecal transplant trials are all currently underway.
Mitochondria Rejuvenation
The authors pointed to the partial success of AXA1125 in improving Chalder Fatigue scores in long COVID, but it’s clear that, while AXA1125 may help, much more is needed in this direction. The suggestions regarding mitochondrial rejuvenation were surprisingly sparse but interesting.
The authors pointed to NAD+ and/or its precursors (nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN)) supplementation to combat NAD+ declines seen in aging, and reported that NAD+ declines are “a salient feature” of ME/CFS. Nicotinamide riboside helped remove damaged mitochondria, reduce tau protein levels, and improve neuroplasticity in the brains of a mouse model of Alzheimer’s.
Urolithin-A is a really interesting metabolite produced by the (uh oh) gut bacteria. It’s been shown to improve mitochondria functioning, clean out old and inflamed mitochondria, reduce neuroinflammation, reverse cognitive decline, improve muscle functioning, and prolong live span in laboratory animals.
A recent study found that urolithin-A was able to reduce inflammation and improve the clean-up processes in the brains of an Alzheimer’s mouse model. A blog on urolithin-A is coming up.
That was it for mitochondrial rejuvenation.
Bye-Bye Zombie Cells – the Senotherapeutics Option

Zombie cells hang around past their normal lifespan, emitting pro-inflammatory substances. Senotheraputics treatments aim to get rid of them.
As we age, we tend to accumulate more and more senescent, mostly non-functioning cells (aka “Zombie cells”) that emit inflammatory factors, break down the “extracellular matrix” (connective tissue!), contribute to telomere degradation (a sign of aging found in ME/CFS), and contribute to osteoporosis.
Finding ways to clean out these cells and reduce inflammation has become a major focus of anti-aging therapies, and senolytic, or senotherapeutic, treatments are being trialed in a wide variety of disease.
Osteoporosis is, of course, a major concern in chronic diseases like ME/CFS and FM which primarily affect women and make exercise difficult. A recent Mayo study suggested the researchers are on track to help out at least some women with osteoporosis employing a cancer drug called dasatinib and quercetin, which produced increased bone growth but only at 2 and 4 weeks. It worked best on women with high rates of senescent cells.
Studies suggest that fisetin, a flavonoid polyphenol, navitoclax (ABT-263), famotidine (a mast cell inhibitor), suramin, CXA-10, BMS-986301, epigallocatechin gallate (EGGG), tucatinib, anti-diabetic drugs (acarbose, SGLT-2 inhibitors), new anti-obesity medications (GLP-1 receptor agonists (e.g., semaglutide) and dual GLP-1/GIP agonists (e.g., tirzepatide, lixisenatide) can all help remove senescent cells. Interestingly, hyperbaric oxygen therapy (HBOT) is a possibility as well.
Rapamycin/Rapalogs and Metformin – Enhancing Autophagy and other Processes
Autophagy is another garbage clearing process, but in contrast to cells, it refers to cellular components. If damaged organelles – in particular, the mitochondria – are not cleared out, the cell will suffer.
Metformin has been shown in some studies to increase lifespan and protect against age-related disorders probably through its ability to improve autophagy and thus reduce inflammation. It also appears to be able to increase gut diversity, and, interestingly, reduce the risk of long COVID.
Rapamycin’s ability to extend the lifespan in several animal models helped to jumpstart the longevity field. Rapamycin’s ability to do that appears to rest in on its ability to reduce the number of exhausted T-cells (a clear problem in ME/CFS), tone down MTORC1 activation, increase gut diversity, reduce inflammation and enhance autophagy. Resveratrol, spermidine and again fisetin may also be able to enhance autophagy.
A Rapamycin ME/CFS trial is underway under the auspices of the Simmaron Research Foundation.
Signs of Accelerated Aging in ME/CFS
Some studies have explicitly stated finding signs of accelerated aging in ME/CFS. A 2018 CDC study found evidence of teleomere reduction that translated to roughly 10.1–20.5, 4.0–8.2 and 6.6–13.7 years of additional aging depending on the degree of ME/CFS (full, almost ME/CFS, fatigued). Back in 2013, Dr. Newton stated that she felt the ME/CFS could benefit from studying aging processes. Dr. Klimas recently reported that the T-cells in ME/CFS patients look like they’ve aged 16 years. A muscle study proposed that the muscles in people with ME/CFS had prematurely aged. The title of one paper “Endothelial Senescence and Chronic Fatigue Syndrome, a COVID-19 Based Hypothesis” said it all about the possibility that the blood vessels in ME/CFS are aging. Fatigue in older adults was associated with basal ganglia dysfunction – which is found in ME/CFS and probably FM as well.
The Homeostatic Diseases
The authors ended by describing long COVID as a disease in which homeostasis – the ability to maintain biological stability – is under attack. When the body is in homeostasis, the system responds to a stressor – and may bend under the strain – but then snaps back to normality.
The authors refer to this ability to snap back as resilience or “physiological reserve”, which wanes as we age. Simply put, the body lacks the ability to recover completely from, in the case of long COVID and many people with ME/CFS, an infection. Something that happens during the infection impairs “the biological mechanisms of resilience”.
While aging could be described as a disease of disrupted homeostasis on virtually every level, ME/CFS, too, has been viewed as a disease of impaired homeostasis. Nancy Klimas has been the foremost champion of the idea that a stressor (usually an infection) has pushed ME/CFS patients into an alternate steady state from which it’s difficult to escape. The idea, though, goes way back.
In 1994, Gray and Martinovic proposed that problems with fatty acid metabolism caused “many homeostatic system(s) (to) become deranged in ME/CFS and held in that state by minor stressors.” Niblett (2007) proposed that “urinary excretion and blood parameters data supported the hypothesis that alterations in physiologic homeostasis exist in CFS patients.”
Gupta’s 2009 model proposed that stress to the HPA axis had forced it into an “alternate steady state” that prevented it from returning to homeostasis. Reuter echoed Gray and Marinovic when in 2011 he proposed that “carnitine homeostasis” was disturbed.
Barnden moved the homeostasis idea to the brain in 2011 when he proposed that an “insult to the midbrain” which “affects multiple feedback control loops” “disrupt(ed) local central nervous system homeostasis“.
Nancy Klimas’s group’s 2014 paper, “A role for homeostatic drive in the perpetuation of complex chronic illness: Gulf War Illness and chronic fatigue syndrome”, proposed that men with ME/CFS were in an “alternative steady state” characterized by hypercortisolism, low testosterone, and a shift towards a Th1 immune response. Women, on the other hand, were caught in an “alternate homeostatic state” characterized by hypocortisolism, high estradiol, and a shift towards an anti-inflammatory Th2 activation.
In 2018, Germain and Maureen Hanson found “a disturbance in homeostasis of metabolic networks“. In 2022, they noted that the “numerous altered pathways” present depended on glutamate metabolism, which they called “a crucial component of the homeostasis of many organs in the body”. In 2023, Marks used the “central homeostasis network” in the brain to propose that the “dyshomeostasis” in ME/CFS and long COVID is “caused by a chronic state of multisystemic disequilibrium including endocrinological, immunological, and/or metabolic changes.”
Note how often the metabolism and the stress response figure in these hypotheses.
The Upside – Turning Back the Biological Clock in ME/CFS, Long COVID and Allied Diseases?
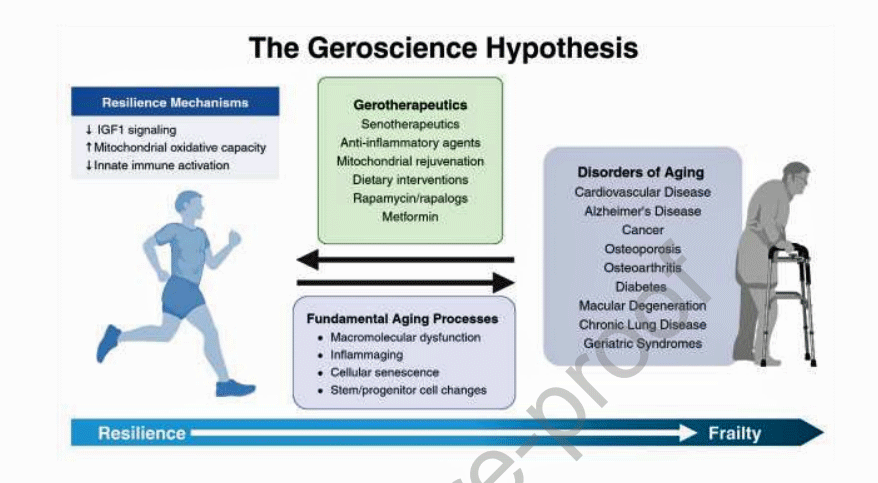
The Geroscience Hypothesis from Shafqat et Al.
While it’s a bit wrenching to think that, on top of all the other stuff going on in these diseases, one might also be biologically aging more rapidly, the good news in all this is that the longevity, or geroscience, field is booming.
The “Interventions Testing Program (ITP)” at the National Institute of Health (NIH) is dedicated to identifying the treatments that extend the health and lifespan of mice, and should be explored further. (While mice are not humans, note that mice studies are often the entryway to human trials.)
Thus far, the ITP has identified dozens of interventions that might be able to turn back one’s biological clock. Included are some familiar (rapamycin, fish oil, resveratrol, nicotinamide riboside (NR), methylene blue) ones as well as many I’ve never heard of.
Billions of dollars are going to fund longevity research every year. If diseases like ME/CFS and long COVID exhibit accelerated biological aging, they should benefit from the increasing interest in longevity. Three billion dollars, for instance, has been invested in a company focused on cellular regeneration called Altos Labs. Altos Labs’s explanation of its work sounds like it might relate to a disease characterized by low resilience and difficulty responding to stress.
“Cells in healthy, resilient states resist stressors that give rise to disease, but this diminishes with aging. Early experiments have shown that this ability to resist stressors can be restored.”
It was nice to see a long journal article dedicated to explicating the similarities between aging, ME/CFS and long COVID, and potentially providing some new treatment options such as some immunotherapies and senotherapeutics. We don’t know if diseases like ME/CFS and long COVID are causing accelerated aging, but if so, they stand to benefit from the interest in aging research.
Health Rising’s Quickie Summer Drive Update

Will longevity treatments hit the mark? Time will tell.
Thanks to the over 180 people who have brought us about 60% of the way to our goal 🙂
This is Health Rising’s first blog on longevity research but with that field exploding and with so many biological similarities between aging and ME/CFS, long COVID and allied diseases showing up it won’t be last. We’re committed to keeping on top of all possibilites.
If you appreciate our attention to this (and other) new areas please support us!
Please Support Health Rising and Keep the Information Flowing






Don’t forget this study
Association of chronic fatigue syndrome with premature telomere attrition
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5830066/
This accelerated aging process is quite scary
Yes, I had it in there but updated when I looked at it again. It was quite something! And not something we expected from the CDC.
It found shorter telomere length translated to roughly 10.1–20.5, 4.0–8.2 and 6.6–13.7 years of additional aging in full ME/CFS, almost ME/CFS and fatigued patients compared to healthy controls – but only in younger patients (<45 years) oddly enough
Thx Cort. I think this is in line with the findings of Jason et al. 2007
Although statistically it is not completely justified. These are strong indications. A red flag. ME/CFS patients also die significantly younger from certain diseases.
https://www.tandfonline.com/doi/abs/10.1080/07399330600803766
I’ve been saying this for years cort.
You should interview Liz Parrish.
Shes been trying to heal her son’s type 1 diabetes.
By proxy that led to her setting up a start up in the regenerative medicine field.
She has numerous protocols ( all down in Panama).
She started the experiment s on herself..using MRI imaging to watch for any changes
.her telomeres relengthened, her metabolic markers went to those of a twenty five years old ( shes in her 50s) and most importantly her muscle mass regrew. I mean that could help so many of us who are deconditioning or whose mast cells have ruined our muscles
https://youtu.be/VtPDiT4rgQ4?si=Re8xhp4GbuC2BUBU
Check this lady out. Shes relengthened her telomeres
Hi Cort, I started Rapamycin end of April – there are some others with CFS in an online Rapamycin group, also trying it after reading about the retired doctor on your blog. I don’t feel any better. Found out in this group it can raise lipid and glucose. Seems those who have this side effect then go on statins and metformin. I’m going to go down from 6 to 3 pills as am quite sure it’s causing about 3 days of feeling much worse. Will likely give up on it as who wants to live longer if not feeling well and no energy to do anything. The leader of the group seems interested in the ones on it for CFS and encourages us to post our experiences.
I’m sorry you have not noticed benefit from it. It has truly been a miracle for me. It’s crazy how we all respond differently, I felt so hopeless when I tried other things that have helped others so much and they made me worse. I am in a rapamycin Facebook group, but it is primarily folks seeking longevity. Do you mind sharing the group you are in? Oh and btw, yes I’m able to do so much more, but I still feel lousy myself as well. But the biggest thing that was holding me back was the severe pem, I can deal with feeling lousy and accept that it is what it is for the moment.
Hi Andrea, that’s wonderful it’s helping you! Wish it’d take away the lousy feeling too, wouldn’t it be great to always have a feeling of well being? How much do you take? Has it affected your lipids or blood sugar? The group is a forum that’s part of Rapamycin.news. Maybe you’d want to jump on and tell your success with it. It’s also mostly longevity folks and you’d think quite a few have a PhD in chemistry. They’re very serious about it, lots of blood work, graphs etc. In your other post you said you had a hard time getting a doc to prescribe – they have a section on where to buy. Mine came from China via a company in the UK – most of these folks are getting it from India. They get blood work to see how much is in their blood.
From some brief research it looks like Walnuts, Almonds, and Pomegranate are all good natural sources of Urolithin-A
Yes, dietary sources are so very important, especially for the gut also.
Instead of relying wholly on man-made drugs to treat us, we should be looking more towards nature, like the foods that we eat, etc. (I especially like the ‘healthy’ mediterranean style of eating).
I am not totally against drugs, but they are so very overused in the health system today. Doctors are taught that a certain drug will ‘fix’ a particular health problem and if not, they try another drug, and so on. And unfortunately, they have become solely dependent on them to heal everything in this modern world! But we know…and they know that this simply isn’t true.
PS. And yes Cort, I had those same feelings when a specialist had me step onto a treadmill years ago.
He even said to me that he had 90+ year old patients who were in much better shape than me! That made me feel really good… 🙂
PPS. No need to tell you that to his horror, I collapsed as soon as I got on…
Yes, these are good sources but many people lack the correct gut bacteria to convert it to Urolithin a. At least that’s what I have read ….
Interesting. Did you read that many people with these conditions lack that bacteria or just in the general population? Also do you know which bacteria it is? Or if probiotics could possibly help? The devils always in the details isn’t it!
It’s interesting, I am 52 and have had CFS since age 20 (albeit a fairly mild form since age 23-24, after it was moderately bad age 20-23). Yet many people think, from looking at me, that I am in my early to mid 40s.
Likewise – I am frequently told that I look significantly younger than my age (62) and also that I look healthy (which doesn’t help when I’m trying to explain how tired I feel 😉 ). I have wondered whether the explanation might be that only some biochemical pathways/biological processes are disrupted in ME/CFS, whereas many are not, and because we frequently rest to allow the disrupted pathways to recover, the normal pathways are given much more rest than required and therefore function much better than average (?). Just conjecture with no evidence!
Hi George,
I have always looked younger than my age and I remember Dr. Paul Cheney saying that his patients all looked younger. Maybe it is all the rest or a different genetic makeup, I don’t know. But since he saw thousands of patients, I would rely on his observations rather than “another” study.
I mentioned “ looking younger” than my age to a doctor when I first got sick and that I was suddenly and rapidly looking older …..She said people with EDS always look younger…… come to find out she was right in my case !!! I had no idea !
Navieux has also commented on the state of health for those who do recover being very good despite years of illness. I think he hypothesized it being due to the arrested state of the cell danger response?
Whenever I go into a partial or temporary remission, I’ve been told I look younger than my age too. But I think in the midst of a crash I look pretty shot out 😉
I think it applies to the general population….the bacteria hasn’t been identified.
Oh….I should add that Mitopure is a Urolithin- a supplement !.
Thank Cort, nice article.
Diseased, degenerating cells, ‘suffocated’ by a lack of oxygen due to toxins, viruses, heavy metals, bacteria….?
If this is the case, we would therefore need therapies that allow the body to self-repair and self-regenerate, such as high-dose IV ozone therapy, which, according to this study, has given good results on fatigue in ME/CFS.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8745272/
Anyone wanting to take a deep dive into the anti-aging field, could start by doing a search on YouTube for the “Found My Fitness” Channel. It has many videos hosted by “Dr Rhonda Patrick” – an anti-aging researcher that has now shifted to being a medical journalist in this field. Sometimes, she does video interviews with other anti-aging researchers about the findings of their research. Other times she explains new or pertinent research (kind of like a “Cort” of the anti-aging field … lol). Her recommendations and suggestions are very scientifically based. Sometimes the explanations really get into the weeds.
However – fair warning – a very big emphasis is placed on the benefits of exercise (and the how’s and why’s of how it decreases aging). However, they also talk about different (generally nutrient dense and low carb) foods that can also reduce the rate of aging, as well as fasting, and exposure to cold (or heat) as stressors that increase resilience. She recommends a green smoothie – made from fruits and veggies from the grocery store, and explains the anti-aging role of each one. (Search for her “Green Smoothie 1” for an explanation of the science, and “Green smoothie 2” for an updated recipe).
There are probably many other topics that have been discussed there now, as I have not looked at this channel for a few years. I lost my enthusiasm after I followed it closely for a few years when I realized that almost everything they suggested – I couldn’t do (with the reduced energy and increased fragility of my CFS and Fibromyalgia). Perhaps that might contribute to why we age faster?
I remember telling my Doc (decades ago); “You don’t have to encourage me to exercise. You just need to enable me … and then just try to stop me!”.
I have been on rapamycin/sirolimus over 6 months now and it was a miracle the first week of trying it. I feel like it has saved my life, but having difficulty getting it prescribed since I moved. Practitioners don’t care to educate themselves on how it can help someone with me/cfs/lc even with me explaining the horrific loss of life I experienced and the remarkable increase in life I have gained through this medication. I sure hope more studies will be done!
That is great to hear! Dr. Kaufman on Unraveled said he thought it could be quite helpful. Hopefully the Simmaron Research study gets good results and we can go onto a good placebo-controlled trial.
in all this field that is exiting for everyboddy and so many deseases, lets not forget that there is worldwide almost no money for ME/cfs research. there is more for LC and much more for other diseases. So i expect other deseases will profit from it but for ME/cfs, you know… Otherwise there was much more money for other studys for ME/cfs… by the way, i feel 12O, is that possible? 🙂
Feeling like 120 is certainly possible and I’m sure you are not alone. Right now I feel like I guess a 85 year old on a bad day would feel like. 🙁
I think you are also to long ill 🙂 or my passed away dad was an excemtion… in his 80 he coocked, he took care of me, he washed, he did grocerys, he drove from belgium to germany to do buiseness, he painted the house, he did so much more … I think if we where suddenly cured, we could not get it what we all can do more on every age (I mean i am getting old anyway now…) But you feeling 85 is still a way younger then 120! hope you feel better tomorrow if possible!!!!
Thanks, Konjin! That was a low point….feeling better today. Thanks very much! I hope you are as well!
I am glad you are feeling better!!! 🙂 here even worse 🙁 must rest and rest and rest in dark room but everytime there is a problem, little things i can not avoid being alone… for excample could have slept evening, night, day but got to wake up for help. and that is the least… stay well!!!
See re Dr. Jonathan An, U Washington School of Dentistry, studying (and using) rapamycin for gum disease in patients over 50. Doses for our purposes are small: one doctor in NPR story on July 1 mentioned 6 mg every Sunday for 3 weeks then a month off then resume 6 mg every Sunday etc. His shoulder pain went away. Doses that are much higher are for use in organ transplant patients – not recommended for the general population!
They are also starting up a study on dogs using rapamycin to see whether this can lengthen the life of dogs. I believe this is also coming out of the University of Washington. See NPR story Jul 1 2024 Morning Edition.
Cort,
Have you heard of or researched Dr. Pompa’s cellular inflation program? Since we are hearing from so many sources about inflammation in CFS/ME/FM I was wondering if anyone has actual experience with his program?
https://pompaworkshop.wpenginepowered.com/presentation/#gf_1
It’s inflammation not inflation as mis-typed…
Inflation – ha! ha! – Gave me a laugh :). Thanks – I had not heard of Dr. Pompa – will check it out!
My Partner, who is 66 years old, was diagnosed with Parkinson’s disease last year. We noticed that he was experiencing hallucinations, slow movement, disturbed sleep, and twitchy hands and legs when at rest. He had to stop taking pramipexole (Sifrol), carbidopa/levodopa, and 2 mg of biperiden because of side effects. Our family doctor recommended a PD-5 treatment from natural herbs centre , which my husband has been undergoing for several months now. Exercise has been very beneficial. He has shown great improvement with the treatment thus far. He is more active now, does more, and feels less apathetic. He has more energy and can do more activities in a day than he did before. As far as tremors I observe a progress, he improved drastically. I thought I would share my husband’s story in case it could be helpful, but ultimately you have to figure out what works best for you. Salutations and well wishes
Thanks so much for sharing. It’s always so good to hear when natural treatments help more 🙂
I’ve been saying this for years cort.
You should interview Liz Parrish.
Shes been trying to heal her son’s type 1 diabetes.
By proxy that led to her setting up a start up in the regenerative medicine field.
She has numerous protocols ( all down in Panama).
She started the experiment s on herself..using MRI imaging to watch for any changes
.her telomeres relengthened, her metabolic markers went to those of a twenty five years old ( shes in her 50s) and most importantly her muscle mass regrew. I mean that could help so many of us who are deconditioning or whose mast cells have ruined our muscles
I started a low dose of NAD+ last week. Just 1 cc. It kicked in in a few hours and I had a notable increase in energy. But context is important to share.. I’m severe and bed/couch bound, but ambulatory.
I’m also going through IVIG treatment. IVIG will knock me out for a day or two and the NAD+ was enough to lessen that. On days where I could not do a quick sponge bath, the NAD+ allowed me to.
My husband ordered it from AgelessRx