

Geoff’s Narrations
The GIST
The Blog

The study showed that pathogenic triggers in the brain impacted the muscles ability to do work.
It’s always seemed that both the brain and the muscles must be involved in diseases like chronic fatigue syndrome (ME/CFS), fibromyalgia (FM), and long COVID. The question has always been how? A study just showed up could answer that question.
The problem starts in the brain, is triggered by an infection or a toxin that produces neuroinflammation, and somehow finds a way to impact the ability of the muscles to produce energy (!).
The new finding has birthed something called the brain-muscle axis. First, though, we look at the opposite: the muscle-brain axis.
THE GIST
- It’s always seemed that both the brain and the muscles must be involved in diseases like chronic fatigue syndrome (ME/CFS), fibromyalgia (FM), and long COVID. The question has always been how? A study just showed up could answer that question.
- It originates in the brain, can be triggered by infection, is associated with neuroinflammation, and impacts the ability of the muscles to produce energy (!). The new finding has birthed something called the brain-muscle axis.
- We’ve known for decades that the muscles are sending messages to the brain and affect things like energy metabolism, glucose metabolism, inflammation and bone formation.
- In this study, the authors introduced a variety of triggers (bacterial infections, the SARS-CoV-2 protein ORF3a, and the neurotoxic protein Aβ42 found in Alzheimer’s) to produce neuroinflammation in the brains of the ubiquitous fruit fly and mice.
- Note the interesting disease choices – Alzheimer’s and long COVID – two diseases that are rarely mentioned together but both of which feature what the senior author of the study called “deep muscle fatigue“.
- Each animal responded to the introduction of these substances with a clear increase in muscle fatigue and reduced endurance. Introducing these stressors into the brain triggered increased levels of reactive oxygen species (ROS), or free radicals which then triggered the production of the IL-6 cytokine, which then found its way to the muscles, where it turned on genes that turned down energy production.
- For the first time, inflammation in the brain was shown to reduce muscle mitochondrial activity and endurance dramatically. No exercise was needed to shut down the muscles. All it took was neuroinflammation.
- Noting that a lack of motivation does not play a role in this process, the senior author of the study stated: “This is more than a lack of motivation to move because we don’t feel well. These processes reduce energy levels in skeletal muscle, decreasing the capacity to move and function normally,”.
- While the study was done in fruit flies and mice, note that this pathway appears to be highly evolutionarily conserved; i.e. this core pathway is present in many species.
- Noting the large energy needs of the muscles, the authors proposed that when the brain is inflamed it may limit energy production by the muscles to give the brain more resources.
- The two key factors needed for this process to occur – neuroinflammation and increased IL-6 levels – have been found in ME/CFS, fibromyalgia, and long COVID.
- The authors proposed that IL-6 and Stat inhibitors could help, Avindra Nath has also proposed using JAK-STAT inhibitors in ME/CFS.
- We don’t know if this process is occurring in ME/CFS, FM and long COVID, but one suspects that whatever ends up causing these diseases will impact an evolutionarily conserved core pathway that can dramatically affect functionality.
- The good news is that a way to explain how neuroinflammation can directly impact energy production in the muscles has been found. The finding has received a lot of attention, and will undoubtedly hook in some Alzheimer’s researchers, and will undoubtedly be followed up on. The long-COVID/ME/CFS/fibromyalgia research world just got a bit richer and is bringing new potential treatment options to the table.
The Muscle-Brain Axis (???)
We’ve heard a lot about the gut-brain axis, but it turns out, there’s also a muscle-brain axis – and it’s no slouch. In their potentially groundbreaking study, “Infection and chronic disease activate a brain-muscle signaling axis that regulates muscle performance“, Yang et. al point out that we’ve known for decades that the muscles are constantly sending messages to the brain.
Sixty years ago, it also became clear that the muscles were regulating metabolism. By 2021, researchers had identified over 50 muscle-derived proteins that made it, and were impacting the brain. The muscles were pumping out so many compounds, in fact, that they came to be called an “endocrine organ”; i.e. they’re impacting many important processes in the body outside of the muscles – several of which involve energy production – a key issue in these energy-limiting diseases.
That includes increasing the ability of fats to produce energy, lipolysis (fat cell breakdown), glucose homeostasis, insulin secretion, anti-inflammation, angiogenesis, and bone formation. Concerning metabolism, the skeletal muscles have been called “the most critical organ involved in regulating whole-body glucose homeostasis”.
The impact the muscles have on the body as a whole is startling given that it’s always seemed that the muscles must be involved in fibromyalgia (“muscle-fiber” pain) and chronic fatigue syndrome (ME/CFS). We may have just begun to scratch the surface of the role they play in these diseases.
IL-6 – The Jekyll and Hyde Factor
The biggie in the muscle myokine (communication) family is a chemokine called IL-6 that’s produced in astonishing amounts (100-fold increases!) by the muscles during exertion.
IL-6 increases energy levels in the muscle by increasing the outflows of Ca2+ ions, thus enabling them to contract and do work. During exercise, glycogen depletion (the muscle’s energy source) and free radical production (reactive oxygen species) trip the signal to produce more IL-6.
IL-6 then stimulates fatty cells and tissues to release fats, and the liver to release glucose, to provide more energy. (Enhancing muscle-derived IL-6 levels is clearly one reason why exercise is so beneficial.)
IL-6, though, has a dark side. While muscle-derived IL-6 is anti-inflammatory, immune cell-derived IL-6 is associated with inflammation and, even worse, muscle energy depletion.
The study used bacterial, viral, and toxic proteins to stress the central nervous system.
The Study
The authors introduced a variety of triggers (bacterial infections, the SARS-CoV-2 protein ORF3a, and the neurotoxic protein Aβ42 found in Alzheimer’s) to produce neuroinflammation in the brains of the ubiquitous fruit fly, and then moved on to mice.
Note the interesting disease choices – Alzheimer’s and long COVID – two diseases that are rarely mentioned together but both of which feature what the senior author of the study called “deep muscle fatigue“.
“We are interested in understanding the very deep muscle fatigue that is associated with some common illnesses.”
Each animal responded to the introduction of these substances with a clear increase in muscle fatigue and reduced endurance. Determining how that happened resulted in the introduction of a new pathway – the brain-muscle axis.
The Brain-Muscle Axis is Born
We know that in a process called “central fatigue”, the brain affects muscle endurance by activating (or refusing to activate )the muscles, and that the Nath intramural study proposed that pathways in the brain are making exertion more effortful in ME/CFS.
Note how brain-oriented this is: in central fatigue, the brain may or may not be turning on the muscles, but the muscles themselves aren’t damaged.

All it took was neuroinflammation in the brain to whack the muscles.
This study suggested something very different can happen. Introducing these stressors into the brain triggered increased levels of reactive oxygen species (ROS), or free radicals. Lacking an electron in their outer shells, these unbalanced molecules rip electrons out of nearby molecules, producing damage.
The increased free radical levels in the brain triggered the production of the IL-6 cytokine, which then found its way to the muscles, where it activated the Jak-STAT gene transcription pathway.
The genes activated by the Jak-STAT pathway can either help build muscles up or tear them down. In this case, they reduced muscle mitochondrial activity, causing fatigue and reduced endurance. Note that no changes in muscle fiber makeup were found – it was all about energy production.
Thus, the authors reported, the brain-muscle axis was born. In this scenario, the brain is putting the brakes on the muscles but in a more direct way than has been seen before. For the first time, inflammation in the brain was shown to dramatically reduce muscle mitochondrial activity and endurance.
No exercise was needed to shut down the muscles. All it took was neuroinflammation.
While the study was done in fruit flies and mice, note that this pathway appears to be highly evolutionarily conserved; i.e. it’s a core pathway present in many species.
Lack of Functionality – Not Motivation
The authors’ statement regarding motivation echoed what people with ME/CFS have been saying for decades: they’re motivated. They usually have a long list of things they want to do but can’t. Reduced functionality- not motivation – is what is holding things up. The authors couldn’t agree more.
“This is more than a lack of motivation to move because we don’t feel well. These processes reduce energy levels in skeletal muscle, decreasing the capacity to move and function normally,” said Dr. Aaron Johnson, senior author of the study.
Robbing Peter to Pay Paul
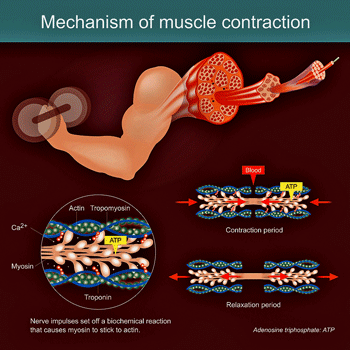
Neuroinflammation impaired the ability of muscles to contract and do work.
Lastly, they suggested why this strange process – the brain taking a veritable 2×4 to the muscle’s ability to produce energy – has been evolutionarily conserved across many organisms. It’s all about freeing up energy when needed.
With the muscles using up almost 30% of the body’s energy production at rest, and the immune system vacuuming up 45% of the body’s energy production during an infection, it makes sense, if neuroinflammation is present, to find a way to free up some energy to help heal the brain.
Turning down energy production in the muscles achieves that while reducing movement – a key part of the process of sickness behavior that results in people isolating themselves when they have an infection.
Mark Davis proposed that a similar process involving the immune system might be working in the opposite direction: the immune system is raiding energy stores and leaving the brain high and dry.
Another possibility is that signals of muscle damage to the brain are causing the brain to shut down the muscles so that they can heal.
Either explanation fits the ME/CFS, fibromyalgia, and long-COVID suite of diseases pretty well.
No Brain Infection Needed
The study demonstrated that it’s not necessary, as many have suspected, for the coronavirus to infect the brain to produce neuroinflammation. All it took, in this case, was for a protein that was able to make it past the blood-brain barrier and persist. All that may be needed to produce a disease like long COVID (or ME/CFS for that matter) are for pieces of the virus to remain.

This study and the Dubbo study suggest that a variety of pathogens (as well as toxins) are able to affect core processes that result in debilitating muscle fatigue.
Many Roads Lead to Rome
The study suggests that a number of different triggers (bacteria, coronavirus protein, neurotoxic protein) produce the same result – a nice finding for a disease like ME/CFS where triggers abound. Indeed, the authors proposed that this process may be present in a variety of infection-triggered, as well as chronic, diseases.
A Long-COVID / ME/CFS / Fibromyalgia Connection
With regard to long COVID, the authors proposed that the increased IL-6 levels found in mild cases of COVID-19 may set the stage for an ongoing activation of the brain-muscle pathway. Their finding that a single coronavirus protein in the brain (ORF3a) can get this process going provided a nice causal pathway for long COVID.
Johnson, the senior author, suggested that once this process gets going, it may become chronic.
“We also see evidence that this effect can become chronic. Even if an infection is cleared quickly, the reduced muscle performance remains many days longer in our experiments.”
Note that the key requirement is neuroinflammation, which Jarred Younger thinks will be wholly accepted in ME/CFS within the next year or so. Studies suggest it is present in fibromyalgia, as well.
The next key factor is an upregulation of IL-6 which has shown to be upregulated in COVID-19 and appears to play a role in fibromyalgia. Note that three types of IL-6 exist: central nervous system, immune system, and muscle-derived IL-6. The first one is both inflammatory and muscle-damaging; the second is inflammatory, and the third is anti-inflammatory.
IL-6 is not a new factor in fibromyalgia or ME/CFS. Several studies suggest that an IL-6 Jak/STAT pathway has been activated in fibromyalgia and that increased IL-6 levels in the cerebrospinal fluid and/or blood play a significant role in FM pain. One study suggests people with FM react more strongly to IL-6 as well. Plus, increased IL-6 levels may be associated with insulin resistance. Increased IL-6 levels have been found in ME/CFS several times but not always.
A long-COVID/ME/CFS study implicated 5 hub proteins, one of which was IL-6, in the two diseases. Increased levels of IL-6 were coupled with reductions in ATP production in long-COVID patients who met the diagnosis of ME/CFS. Pre-pandemic, IL-6 levels were associated with chronic fatigue. IL-6 levels have been found elevated in long COVID several times, and a systematic review concluded that “increased IL-6 correlates with long COVID“.
Treatment Possibilities
“In the meantime, we hope our study encourages more clinical research into this pathway and whether existing treatments that block various parts of it can help the many patients who experience this type of debilitating muscle fatigue.” Aaron Johnson
The authors proposed that IL-6 and Stat inhibitors could help, and mentioned the Stat inhibitor ruxolitinib, which has been approved to treat alopecia, psoriasis, lymphoma, and myelofibrosis.
“These clinical results argue that systemic treatment with IL-6 and JAK inhibitors could inhibit changes to muscle performance induced by the brain-muscle signaling axis and prevent muscle dysfunction associated with chronic and infectious diseases.”
Rob Phair has proposed that Jak-STAT inhibitors be trialed in ME/CFS, and Ely is trialing a Jak inhibitor in his big long COVID trial (thanks Shea and Bailey).
Update! A long-term, severely ill person with ME/CFS quickly recovers using a JAKSTAT inhibitor.
Oddly, despite showing that an “ROS scavenger” (antioxidant) improved muscle performance in the flies with neuroinflammation, the authors didn’t mention using antioxidants to combat oxidative stress in the brain. Theoharides has proposed that mast cell inhibitors can reduce IL-6 levels in fibromyalgia.
A small test of an IL-6 inhibitor, anakinra, however, failed to produce results in ME/CFS.
Conclusion
This study linked the brain and the muscles in a way that’s never been done before. (Image by Gerd Altman – Pixabay)
With their use of different bacterial, viral protein, and neurotoxic triggers, the authors were intent on delineating a new pathway that may be present in many diseases that feature “deep muscle fatigue”.
The study is reminiscent, in a way, of the Dubbo studies in the 1990s which demonstrated that widely differing bacteria and viral infections somehow ended up producing a very similar-looking ME/CFS-like illness. Researchers have been looking to find the core pathway to explain that ever since then.
Could this be it, or part of it? We don’t know, but one suspects that whatever ends up causing these diseases will look something like this. In other words, it will impact an evolutionarily conserved core pathway that can dramatically affect functionality.
Researchers and doctors who are introduced to these diseases are, after all, often shocked by the degree of disability found. Ditto with the inability to engage in what is almost a universal prescription – exercise. Something has gone wrong in a very fundamental way.
How ME/CFS and long COVID – with their post-exertional malaise – fit into all this is, of course, unclear. This pathway, if it does play a role in them, may be more accentuated in these diseases, or there may be add-on effects. Reduced muscle energy production would seem to account for difficulties exercising, but would it account for the post-exertional problems present?
One wonders as well how Paul Wang’s WASF3 ME/CFS finding – which also could explain reductions in muscle energy production – or how Rob Wust’s long-COVID finding – demonstrating that intense exercise caused muscle damage – might fit into this.
However it all fits together, the good news is that a way to explain how neuroinflammation can directly impact energy production in the muscles has been found. The finding has received a lot of attention, and will undoubtedly hook in some Alzheimer’s researchers, and will undoubtedly be followed up on. The long-COVID/ME/CFS/fibromyalgia research world just got a bit richer bringing new potential treatment options to the table. (Thanks to Kristin for the tip about this study.)






This isn’t the first time I’ve heard of Jak-STAT inhibitors being talked about as a potential treatment. In his YouTube video about the Itaconate-Shunt Hypothesis, Rob Phair spoke about the potential of Jak-STAT inhibitors. Cort, have you come across any data (anecdotal or clinical) where that class of drugs was utilized with any success?
Curcumin inhibits Jak-STAT. It also inhibits IL-6. As I have said many times before, curcumin helps me quite significantly with both exercise tolerance as well as PEM.
This is very interesting research!
Btw need curcumin formulations with high levels of bioavailability and which cross the BBB
Could you share your brand and dosage of curcumin please? Thank you
Which curcumin do you Take? Do you have the Product/brand Name?
Yes can you please share your curcumin brand and do you follow instruction dosing? Thank you
I always follow instruction dosing. I have never personally had an issue being oversensitive to supplements and medication.
Thanks. Which brand do you take?
Swanson
As with most things we feel we should try – they are always expensive. 🙁
How much cur cumin do you take?
That’s interesting Matthias as I believe Jarred Younger is about to extend a study he has done with Curcumin as he saw some initial positive responses in an initial study.
I am surprised it hasn’t had more attention over the years. It’s been the only thing that has ever helped me with PEM over many years.
I used longvida in the past, but more recently have used CurQFen. Both are supposed to have higher levels of bioavailability than standard curcumin supplements, and also better at crossing the blood brain barrier.
Btw I haven’t found any benefit from LOLA.
How much did you take and how long did it take until you noticed an improvement in your PEM?
Do you have hypomobility what type tumeric do you take Dr taulbaum.mentions this I have fybromyalgia CFS heds
Jarred Younger’s botanicals study found these three had good results:
Three Microglial Inhibitors That Worked
Curcumin – Pure Encapsulations: low dose – 500 mg; high dose – 1,000 mg; – reduced pain and fatigue; note – it adds some load to the liver.
Stinging Nettle – Nature’s Way: low dose – 400 mg; high dose – 800 mg. (Might stop a virus from replicating.)
Resveratrol – Pure Encapsulations: low dose – 200 mg; high dose – 600 mg). WebMD reports resveratrol may be able to open the blood vessels, reduce clotting, decrease pain and help maintain proper blood sugar levels.
https://www.healthrising.org/blog/2023/01/10/younger-chronic-fatigue-syndrome-neuroinflammation-brain-invasion-psilocybin/
There’s an excellent review on the effectiveness of curcumin/tumeric in reducing IL-6, TNF-alpha and C-reactive protein at this url https://doi.org/10.1016/j.cyto.2023.156144
The conclusions were that all three of these markers were significantly reduced by oral ingestion. In contrast, Il1-beta was not affected by curcumin or tumeric.
I have taken curcumin off and on for 11-12 years and have mentioned it many times in comments here.
I have no idea if it will help others but it has certainly helped me
Thanks for this. I keep searching for something to help prevent PEM. Here is an article on natural products that could be used in brain cancer that have Jak stat action, including curcumin. The idea is to let them reduce the drugs and their toxic side effects. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9526628/
Thank you for this Matthias. I ordered the curcumin last week after all that you wrote here. Being in the UK, the fastest one I could get was Longvida from Time Health. I’ve only been on it for 3 days but it is certainly helping with the breathlessness that I acquired about 5 weeks ago after walking too far on a day out with a friend. I no longer have to lie down after something as simple as a shower.
Interesting about Rob Phair. Haven’t heard anything anecdotal – the first time I heard of JAK-stat inhibitors was probably in the last year.
A total aside here, but every now and again I want to take a moment to thank the fruit flies and mice (et al).
Hahaha
You can’t take your fruit fly for a walk, unlike your dog, but I reckon we are pretty much co-evolving with them as well these days. Thanklessly taking one for the team.
I love it! Absolutely we owe so much to them…
Has anyone read the book, Cure: Your Fatigue by Morley M Robbin’s?
No what does it mention
You would probably need to get the book to fully get the specifics. But it’s about balancing magnesium, iron, copper and ceruloplasmin. He provides tests from an MD to check for the above minerals. Makes a ton of sense.
JAK inhibitors must be the flavor of the month for “everything”. My dermatologist is pushing the topical ointment version for a very rare genetic skin condition I have.
What about hydrogen water to reduce inflammation?
Voici une étude de 2022 sur l’hydrogène et l’EM/SFC .L’hydrogène est un anti inflammatoire et un antioxydant puissant.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9042428/
L’autre article est sur une étude en cours qui se termine dans 3 mois.
https://ctv.veeva.com/study/hydrogen-water-dosing-study-for-me-cfs
J’ai commencé à prendre de l’hydrogène il y a un mois, et j’ai ajouté du Q10 et du fer car mes analyses sanguines sont basses. Je me sens beaucoup plus active avec des périodes de fatigue qui durent moins longtemps, mais je vais faire une pause car mon sommeil est un peu moins bon et je commence à être énervée.
.
Here is a 2022 study on hydrogen and ME/CFS. Hydrogen is a powerful anti-inflammatory and antioxidant.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9042428/
The other article is about an ongoing study that ends in 3 months.
https://ctv.veeva.com/study/hydrogen-water-dosing-study-for-me-cfs
I started taking hydrogen a month ago, and added Q10 and iron because my blood tests are low. I feel much more active with periods of fatigue that last less, but I’m going to take a break because my sleep is a little less good and I’m starting to get annoyed.
Thank you for posting those studies. I started hydrogen water this past month too. I started a lower dose first (1300 ppm) and now higher (3000ppm) although I have not gotten up to 5 bottles per day yet. My bottle has a cannula for inhalation that I haven’t tried yet. It’s definitely helpful. And I have noticed my legs are more consistent in strength (still difficult climbing stairs, but I can do it. I haven’t had any dips in strength where I struggle to step up on a curb)
I notice too if I try to push things too fast for my body, I will be quick to get annoyed.
My neurologist recommended a combination of q10 and cucuramin for fibro.
There is no need to this on fruit fly; it’s easy enough to check IL-6 level in humans. There is no elevated IL-6 in ME/CFS patients (and probably not in LC either). At least not a consistent one. If there was, we would know about it by now.
They had to introduce virus to trigger neuroinflammation. But ME/CFS and LC are post-viral, not viral. I’m afraid they are conflating viral infection with post-viral syndrome. In fact, they said “Infections and neurodegenerative diseases cause inflammation in the brain.” Then they said “This discovery could lead to treatments for muscle wasting in diseases like Alzheimer’s and long COVID”. Such a non sequitur.
Actually as the blog pointed out IL-6 has been found elevated several times in ME/CFS as well as fibromyalgia and long COVID. Not all the time for sure but cytokines are very variable and hard to measure correctly.
I don’t get the non-sequitur comment. The study showed that proteins from pathogens in the brain can damage the muscle’s ability to produce energy.
I don’t understand the viral vs post-viral issue either. A postviral illness has to start with a virus….so in order to understand a postviral illness you have to understand what the virus did to start it off (???)
The keyword, I think, is “consistently”. I’ll have to refer to the large study done by Mary Hornig in Columbia U that found no consistent inflammation in ME/CFS; It only found what appears to be hyperactive immune system at the early stage turning into hypoactivity later on, with no correlation to the symptoms. The LC study seems to be consistent with that finding: their subjects were in early stage.
In the fruit fly study, infection was the direct driver for the neuroinflammation. In ME/CFS, viral infection may trigger the disease, but there is no ongoing viral infection. So, it’s non sequitur to say that you could treat post-viral syndrome with the treatment for viral infection. They are 2 different things. Unless they are saying that neuroinflammation persisted after the virus was gone. But that’s not what they are showed. They simply showed the infection caused neuroinflammation. (“SARS-CoV-2 protein expression caused reactive oxygen species (ROS) to accumulate in the brain”)
Yes agree.
However, I think a study / studies have shown increased cytokines in the CNS?
It’s quite possible there’s neuroinflammation with elevated cytokines in the CNS but generally normal elsewhere in the body.
And it’s still possible of course neuroinflammation isn’t a big thing in CFS, although I strongly suspect it is. I am eagerly awaiting Younger’s research results.
Another possibility is hit and run damage to the brain, that is not a chronic neuroinflammatory phenomenon (although neuroinflammation might be causative).
Once thing I am certain of, and have been for many years – the key issue lies in the brain / CNS
I am waiting for Younger’s results too. But i suspect he didn’t find anything yet because otherwise he would post it on youtube.
I doubt he would have posted it on youtube. Surely he would want to formally publish his results first.
IL-6 is reportedly associated with Long COVID in a systematic review and meta-analysis. https://link.springer.com/article/10.1186/s40249-023-01086-z
“…or at least inform on the “early stage” of long COVID-19.” They found something similar in ME/CFS years ago, with the elevation turning into depression later on. Most importantly, they found that the inflammation did not correlate to symptom severity. That suggests that the inflammation, even when it exists, is not the driver of the symptoms.
Current clinical labs do not test IL-6 levels in the brain itself. We only test peripheral IL-6 which can be misleading when the levels are not high enough to spill over into the blood stream. Testing CSF (cerebral spinal fluid) is invasive, but a few labs do test for this.
As for it being a post viral condition – the body does not need a live virus to trigger an immune and an inflammatory response. That is the whole premise behind vaccines. Just parts of what used to be a live virus can trigger the same response.
Here’s the Rob Phair Jak stat inhibitor video I watched last year on this same thing: https://m.youtube.com/watch?v=PCnkkLlyVMk
Rob Phair too! The list is growing! Nath, Phair and Ely…nice!
I’ve added a couple of references below pointing to IL-6 being elevated in Long COVID patients and the effective use of curcumin and tumeric to reduce Il-6 and other markers of inflammation.
I personally include tumeric with freshly ground black pepper in our daily diet, for example in salad dressings, smoothies, and as a general seasoning in many dishes.
I write about the importance of an anti-inflammatory diet and other LC topics in my posts at Substack, https://longcovidjourney2wellness.substack.com/ Subscriptions are freely available.
Interesting! Ive been adding ground turmeric to “everything” I eat for several years now and I’ve been wondering if these kind of doses have any effect at all or wether I would need supplementation. Maybe I get a half tea spoon a day. Would you or anyone know?
I add about 1/2 teaspoon to all of my salad dressings and more than this to various meat or vegetable dishes. I think that we each consume 1/2 to 1 teaspoon of tumeric each day.
One of my favorite dishes is to add basmati rice to a green salad with an olive oil, balsamic vinegar and Vermont Maple Syrup salad dressing seasoned with tumeric and black pepper, garlic or onions, fresh basil and parsley from my garden.
Just experiment with tumeric. You can even add it to baked goods.
Enjoy!
The science is pretty clear that eating a bit of turmeric everyday will have marginal or no benefit. Curcumin has very poor bioavailability. But certain formulations have been developed that massively increase bioavailability, eg. Longvida, CurQfen
I had surprisingly strong effects from Numi Golden Turmeric Tonic tea (turmeric, lemon verbena and dried lime). I took it for fibrocystic breast. I also had good results with Gaia herbs’ curcumin and piperine supplement (forgot the name of it), and also Theracurmin. I didn’t notice anything from Meriva. Unfortunately I would also get a weird side effect when taking curcumin/turmeric – shoulder pain. It felt the same as when I took Cipro. I checked and both curcumin and Cipro are topoisomerase inhibitors.
https://doi.org/10.1021/bi3014455
Thank you, Cort!
You befittingly write: “How ME/CFS and long COVID – with their post-exertional malaise – fit into all this is, of course, unclear.” This is a lucid interpretation especially as the muscle-brain axis described here does not seem to go along with muscle damage, which seems to be the case in ME/CFS, at least post exertion.
An alternative explanation – at least for the PEM associated muscular dysfunction/damage – could come from Maureen Hanson´s team´s findings ((https://pubmed.ncbi.nlm.nih.gov/38173127/ )): they showed that, in healthy people, exercise causes a rise in several proteins which are thought to protect muscles against damage from exercise as they can induce repair processes in muscle cells (like actin cytoskeletal proteins). According to their work, this surge – obviously typical for the recovery phase of the stress reponse in healthy people – seems to be lacking in ME/CFS patients. This would tie into the many other findings of an abnormal (or, to be more precise, lacking 😉 ) stress response in ME/CFS.
So the process described here (muscle brain axis) may well be a general protective pathway activated during infections in general and probably part of the sickness behavior complex. And yes, it may thus be relevant in ME/CFS, too, which is marked by repetitive endogenous viral reactivations and thus inflammatory stress and immune activation. But this type of muscle shutdown may not be the explanation for the *specific* ME/CFS type of muscular problems: the exercise induced tissue damage which Rob Wüst has shown. Possibly, the work out of Maureen Hanson´s lab may be more relevant for this piece.
Thanks Herbert – I agree that Maureen Hanson’s work showing that the repair processes that should be present after exercise don’t seem to be kicking in in ME/CFS is very telling…
Maybe this is why ME/CFS can be so unusually bad – a problem generating energy in the first place plus the repair process problem.
Time will tell!
Would this make sense to why I’m overweight despite not overeating and eating well?
Ritchie Shoemaker explains this could come fron leptin resistance (see Biotoxin Pathways in survivingmold.com
I’m giving a read now thank you. My inflammation levels are completely off the chart and it’s not auto immune or an infection
And i’m also obese despite not over eating, or eating any processed food and being active.
This peptide PEPITEM looks promising..
https://medicalxpress.com/news/2024-07-naturally-peptide-agent-inflammaging.html?fbclid=IwZXh0bgNhZW0CMTEAAR3zleNjyV_wWw7T7sJjsErIzFbz4sNC-y9_ocRdOJL2_A0uJbN_r_7j1gE_aem_4oz90evLalVvFUny80a8oA
Along this lines, Dr Ely has started a trial using jak stat inhibitor and immunomodulation in LC. The suggestion came from an AI machine learning result.
https://youtu.be/GYi9NJelm2A?si=69O7h3ToJcrYZaz2
Ah! Baricitinab is a jak inhibitor! Thanks! I will put that in the blog. 🙂
am not a medical professional and I am not making a recommendation, just stating what is working for me.
Curcumin is the active ingredient in turmeric.
Turmeric itself contains very little curcumin.
You have to find a supplement with concentrated curcumin.
Curcumin is a strong Cox 2 inhibitor.
Ginger is a strong Cox 2 inhibitor.
Neural inflammation is mostly Cox 1 driven. (Microglia neural immune cells)
“Inhibiting COX-2 could be detrimental in certain neuroinflammatory conditions”.
” animal studies have shown that specific inhibition of COX-1 alone does not alter gastric or intestinal mucosal integrity, and gastrotoxicity was observed when both COX-1 and COX-2 were inhibited”.
My saga
20 plus years ago large doses of aspirin were very helpful for my ME/CFS but couldn’t find out why.
I was hospitalized and 2022 with a relapse, they gave me a one timeToradol injection by IV, a strong Cox 1 inhibitor. I felt like a new man for a while after this injection.
Next I tried Meloxicam at 15 mg and this was very helpful. (Cox 1 selectivity increases with increased dosage).
Next I tried Celebrex a selective Cox 2 inhibitor. This almost made me bed bound again.
Next I tried Piroxicam, more Cox 1 selective. This worked as well as Meloxicam at 15 mg.
Now taking over the counter Naproxen 220 mg three times daily. This was a game changer, much improved but not 100% and not a cure.
Now increasing my dosage of Naproxen to 220 mg four times daily.
This is also working for my macular degeneration caused by the same Cox 1 microglia cell inflammation as a neural
§§§ Is cyclooxygenase‐1 involved in neuroinflammation? (2021 literature review).
§https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9542093/#:~:text=Conclusion%3A%20Taken%20together%2C%20studies%20in,increased%20near%20sites%20of%20inflammation.
§§§ Highly Selective Cyclooxygenase-1 Inhibitors P6 and Mofezolac Counteract Inflammatory State both In Vitro and In Vivo Models of Neuroinflammation (2017).
§ https://www.frontiersin.org/journals/neurology/articles/10.3389/fneur.2017.00251/full.
§§§ Targeting cyclooxygenases-1 and -2 in neuroinflammation: therapeutic implications (2011).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3008299/.
§ “COX-2 genetic deletion or pharmacological inhibition can worsen the response to neuroinflammatory stimuli”.
§ ” On the other hand, the discovery of novel COX-2-derived lipid mediators with pro-resolving properties suggests that inhibiting COX-2 could be detrimental in certain neuroinflammatory conditions”.
§ ” animal studies have shown that specific inhibition of COX-1 alone does not alter gastric or intestinal mucosal integrity, and gastrotoxicity was observed when both COX-1 and COX-2 were inhibited”.
§ ” Therefore, NSAIDs with higher selectivity for COX-1 rather than COX-2 are more likely to reduce neuroinflammation and should be further investigated as a potential therapeutic approach in neurodegenerative diseases with a marked inflammatory component”.
§§§ Novel selective COX-1 inhibitors suppress neuroinflammatory mediators in LPS-stimulated N13 microglial cells (2011).
§ https://www.sciencedirect.com/science/article/abs/pii/S1043661811002714?via%3Dihub
Dennis, this is interesting info. But I’m surprised you are doing well on Naproxen. It is not just a Cox 1 inhibitor, it also inhibits Cox 2. That’s why I haven’t taken it. I was on ibuprofen (Motrin) for years for migraines, and it always helped. Although I only took it 2-3 times a month, it really seemed to help with my Fibro too. However it causes gastritis for me often, so I stopped taking it a few years ago. I realized that ever since I stopped taking it my Fibro has gotten worse. But I also had Covid twice and now have Long Covid. So not really sure what is triggering everything. I do feel that Cox 1 inhibitors could be helpful, but didn’t realize there were Cox 1 meds available. Why did you stop the other Cox 1 meds you mentioned?
Cox 1 inhibitors control inflammation caused by microglia immune cells found in only these two places, the CNS and behind the eye retina. Researcher Jared younger believes brain information is the cause of the symptoms for ME/CFS, long covid, fibro and Gulf war illness. Cox 1 is also a blood vessel constrictor and Cox 2 relaxes blood vessel.
All cox inhibitors inhibit both 1 and 2 but very in their degree of selectivity. Apparently there is no way to submit images on these posts, I have images that list in order the selectivity of Cox inhibitors. Naproxen is one of the least selective of Cox 2 OTC meds, thus more selective of cox 1.
Ibuprofen is in the middle of the chart with a somewhat equal selectivity of Cox 1 and 2. If 1 and 2 are both inhibited it is more likely to cause gastric problems.
Meloxicam a prescription medication has a good safety profile and may not cause the same problem. With meloxicam, the higher the dose the more selective of Cox 1 (the proper way to say this is less selective of cox 2). You may want to try this at 15 mg, not 7.5 mg but naproxen is OTC.
Good luck Robin, I would love to know how things work out for you.
People with fibro …..please research THIAMINE
there is a guy by the name of Elliott on youtube that has a video on fibro and how high dose THIAMINE is making fibro go away.
Then read the comments below his video….many people fibro goes away….along with nasal stuffiness etc etc
I had a lot of chronic pain – not sure if it was from fibro, myofascial pain or small fiber neuropathy – no dx.
TTFD has helped me enormously. I tried benfotiamine 1st, and it helped, but I had some side effects that suggested increased prolactin (breast tenderness, etc.), so I switched to TTFD. I had been taking a B-complex with thiamine Hcl for many months and it did not do anything for my pain. I had also tried high dose thiamine Hcl in the past and reacted with rashes – though that could have been due to corn-based excipient (I have since been dx with corn allergy). Another thing that helped with pain is an elimination diet. I found this out by accident when I went on a colonoscopy prep diet. The second day I woke up in no pain for the 1st time in a long time. I was just eating rice, tapioca (made with water and milk only) and apple sauce.
https://youtu.be/FSh_5AVXVsg?si=cHYys5uQ117-UW08
☝️peeps…please have a listen to this guy.
He has helped a lot of people with me/cfs, fibro
Just by simply suplimenting different types of thiamine
CRAZY!!!
he has a few videos on youtube on this subject…..with interviews of people testimonials.his name is Elliott Overton
Thank you for publishing this information. I am a Long Covid survivor having had Covid in 2020. All along I have been telling doctors that the issue was in the brain.
Below is a link to an interesting talk on sepsis causing acquired Na channelopathy, resulting in critical illness myopathy and even coma. Something like this could be at work in ME if we consider both ME and sepsis related coma to be extreme forms of sickness behavior.
https://youtu.be/Ypc9vkf-vCI?si=Um2Z6LJt0nd7aG2P
I honestly believe I went septic here at my home.
I had no idea a person could become as sick as I was. Pretty sure I was close to death.my bedroom smelled like burning rubber tires….all coming out of my skin
…my bedsheets would be very brown stained in only a couple days….one hellova ride…now I have PTSD from all of this.
I am not an expert on detoxing but what you describe sounds like detoxing. Your body was getting rid of a lot of stuff that it did not need or want. I don’t know what could have precipitated the detox, and why it was so violent, but in the long run it could have been a very positive thing (though very worrying in the short run). Did you feel better once you recovered? And if you did, perhaps you could look at some more gentle detoxing using far infrared sauna.
Hi, I wouldn’t call my present state “recovered” but I’m better than I once was.
My story started with an “infection”
In a motel room as I was living there for 5 months going to welding school.
But the motel was secluded on a hyway and was using well water….this long before water testing (1981)
Two weeks after that illness I developed an ulcer…the ulcer healed on its own,…the symptoms just kept piling up over the years until
KERBOOM! what I believe to be my body trying to rid itself of this infection…….I started fasting and that’s when my body healed but a week later I fell apart once more and I’ve been stuck ever since
Wow! Your body really worked hard to rid itself of that infection. I hope you can find something that helps.
I ordered longvida curcumin after reading the comments above. Today was my first day on it. Interestingly, it is the first day in weeks when I have not had an attack of being short of breath from doing too much. Lately I’ve had to lie down after a shower, but not today. I’m looking forward to seeing how much it will help in the days ahead.
I am injured after one Covid jab. I have been using turmeric and black pepper supplements for two years. I ran out recently and noticed the difference with pain. It really does help. Red light therapy and methylene blue also help with mitochondrial support. Listen to The Red Light Podcast on Spotify.
Go check out Dr. Andreas Kalcker on Rumble.
It would be great if everyone with CFS would look into Andreas Kalckers protocol and then check back with their results if they try it.
I’m doing it now and if it helps me I will come back and give a report.
Please do, but post somewhere other than one of these blogs as it would be almost impossible to find it.
I know a couple people that claim to have cured cancer with his protocols
This theory adds further weight to what I’ve experienced, particularly in recovering.
When I read that inflammation was an issue in fibro many years ago I started studying that and identifying every part of my body and life that was pro-inflammatory. At the time I had severe gut issues so that was the obvious place to start. Turned out I had Helicobactor Pylori which had done serious damage to my whole GI tract. I made a safe assumption I had ‘gut permeability and read that BBB permeability often accompanies that. I set about healing my gut. When I removed gluten and dairy (known to cause systemic inflammation particularly with leaky gut), my symptoms got significantly better.
I was also experiencing chronic herpes flares so knew I needed to get on top of that using antivirals.
I was always an athletic type person and in my 20 years of severe fibro pain I always exercised but paid for it with increased pain. I wanted to exercise, I enjoyed exercise and I certainly wasn’t deconditioned but my body reacted with severe pain.
Once I healed my gut, identified and removed inflammatory foods, reduced oxidative stress, loaded up on antioxidants and anti-inflammatories, prioritised quality sleep and stabilised my blood sugar I was able to increase my exercise without flares or pain.
I now work out at the gym 4 times a week with zero pain the next day. In fact, unless I slip up or get exposed to something I am living pain free almost all of the time.
I wonder if the a contributing part of the post-viral syndromes is the impact on the gut and subsequent leaky gut and leaky BBB. If that is happening then ingesting food proteins everyday can drive immune reactivation and neuroinflammation.
I’ve found the work of Dr Datis Kharrazian to be particularly brilliant in explaining these mechanisms as he links gut function – immune function – neurology – endocrinology. He has an online gut course and an Immune Tolerance course that helped me immensely.
Interesting stuff.
Since the origin of the IL-6 makes such a big difference to which action it has in the body, do we know if IL-6 inhibitors act on all 3 types? Is there a possibility that we need to have a specific type of IL-6 inhibitor to do the job?
Senescent cells could be driving neuro inflammation MECFS and LC patients.
Perhaps in genetically susceptible individuals, viral infection causes damage to cells in CNS, muscle, endothelium, etc leading to senescent cells that are pro inflammatory (especially when stressed). Perhaps cytotoxic T cells are unable to clear these cells (T cell exhaustion).
Could a PET tracer be developed to look at cell surface markers expressed in senescent cells of MECFS patients vs age matched controls? Perhaps Dr Michelle James at Standard could explore senescence cell marker tracers for MECFS?
Could therapy to rid the body of senescent cells be used as a treatment for MECFS?
Hi, my name is Dr. Keith J. Cronin and I would be very interested in speaking with authors of this article as I put out a White paper a month ago specific to this topic with the use of whole body electrical muscle stimulation to address this axis. We have seen benefits with seven out of seven patients we’ve used this with for long Covid, as well as hundreds of others with autonomic nervous system dysfunction. This falls directly in line with what we are doing at Neuro20 and as it happens, I am doing the a presentation on electrophysiologic neuromodulation this Wednesday July 31st as a webinar. This is exceptionally exciting and I’m glad to see that the science is coming together to help people with these very difficult conditions.
I take bio available Curcumin which i think helps some, but has no life changing impact on my level of disability (fatigue, muscle pain and weakness, PEM). This is not an unsafe supplement-can’t we just assume that most MECFS patients should try it? I hate to hear of science dollars going to test something like curcumin that so many of us are already using and which is benign and easily available. Science dollars should go towards finding a cure rather than to feel slightly less discomfort.. Unless the research proposal is to remarkably change the dose, such as quadruple what we are currently taking to get better results.
I believe these findings explain why purely cognitive exertion causes muscle weakness/stiffness/pain for me, as well as why I can come down with severe full body weakness overnight due to PEM, and recover from it just as quickly overnight following aggressive rest. I’ve been trying to tell people for years that it’s neurological and not caused by deconditioning or physical damage to the muscles. Nobody believes me
How does this relate to the chronic pain suffered? I have a theory but dont want to presume.
Nah, this info and study has been done during(CFS) STAGE.. SO WE HAD TO GO THROUGH FIBRO,ME,NOW LONG COVID/ to get 11billion you say?Also the label(trivialized CFS, originally) to only get 1 million in research! Still after 34 yrs. I feel again they are pushing CFS SUFFERERS again under the rug! WILL someone please tell us what and how it happens!! At 68 now, aging factor kicking in..Thanks for digging up also adding on the obvious.Some still want to and need to hear before checking out after 1/2 lifetime, dealing with 😵💫🙈🙉🙊. “They know”… Wouldn’t wish this on anyone! “sick and tired of being sick and tired!”The “Mack Truck” which way did it go, didn’t see it coming.SYNDROME. 🙄🙏😑. The simple cold that wouldn’t leave..(remember?)Sincerest Blessings to all.
hi Cort –
As a long haul sufferer of mysterious intermittent weekly Fog, CFS and Exercise Intolerance I hit a couple of rough patches in the last two years, the most recent of which had me flat on my back for most of the last 3 months and unable to function. On a scale of 1-10, my gas tank hovered around a relentless 3-4. At times I felt like I was one breath away from my last. After years of experimenting with every drug and supplement without any effect – I seized on a friend’s report of his partner’s chemo cocktail from which she felt improved energy. After learning that she was given dexamethasone – I managed to get my doc to give me an experimental dose with which I have been experimenting. Initial results – after two weeks of mostly 2mg a day my energy has significantly recovered to a functional 6-7ish. Although I now have to contend with my endocrinologist who will likely want me to stop it – I wonder if you have ever encounter similar such reports of its use for FATIGUE – even if just as a temporary emergency aid? Thanks for all you do.
Are there any updates on the Wes Ely Baricitinib Trial? That would be related to all this?
Here is the link to the listing for Dr Ely’s study on barcitinib for Long COVID:
https://clinicaltrials.gov/study/NCT06631287
It’s at four locations in the USA and seems to still be recruiting:
https://clinicaltrials.gov/study/NCT06631287?term=NCT06631287&rank=1#contacts-and-locations