Geoff’s Narrations
The GIST
The Blog
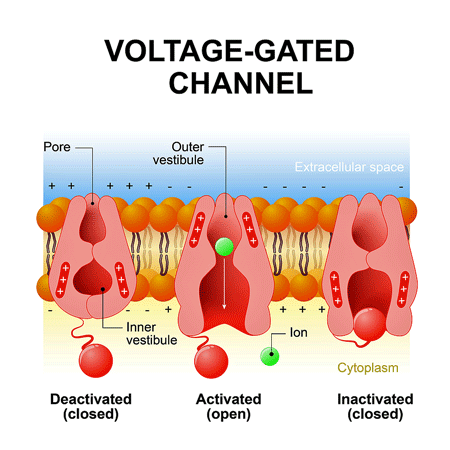
A voltage gated ion channel. The channel needs to open for ions to get through to the cell.
The “transient receptor potential melastatin 3” (better known as TRPM3) saga in chronic fatigue syndrome (ME/CFS) is really something. It’s an example of one group – and only one group – gloaming onto something in ME/CFS and gnawing at it like a dog with a bone.
We’ve seen this kind of single-minded focus before with John Chia and his work with enteroviruses, the Williams-Arriza group, and their focus on the EBV dUTPase protein, and the Barnden group and the brainstem.
Unlike broader areas of ME/CFS research such as metabolomics, cytokines, and exercise physiology these group’s findings have not received as much support from the ME/CFS research field. Each, though, has produced a significant body of research.
No group better illuminates this pattern than Sonja Marshall-Gradisnuk’s work with TRPM3 ion channels at Griffith University in Australia over the past 8 years. (The Griffith group works on other factors in ME/CFS as well.)
Since the publication of the first TRPM3 paper in 2016 (aptly titled “Novel identification and characterization of Transient receptor potential melastatin 3 ion channels…”), the group has published no less than 17 papers on the possibility that disruptions in the TRPM3 (and other TRPM ion channels) are contributing to ME/CFS.
While ion channelopathies were proposed to play a role in ME/CFS as early as 2000, it’s safe to say that TRPM3 channels were on no one’s radar before 2016.
THE GIST
- The “transient receptor potential melastatin 3” (better known as TRPM3) saga in chronic fatigue syndrome (ME/CFS) is an example of one group – and only one group – gloaming onto something in ME/CFS and gnawing at it like a dog with a bone.
- Since the publication of their first TRPM3 paper in 2016 (aptly titled “Novel identification and characterization of Transient receptor potential melastatin 3 ion channels…”), the group has published no less than 17 papers on the possibility that disruptions in the TRPM3 (and other TRPM ion channels) are contributing to ME/CFS.
- The TRPM3 ion channels control the flow of sodium and calcium into the cells and play a role in everything from energy production, muscle contraction, neurotransmitters, cell membrane health, electrical signal generation, fluid balance, etc., and have been implicated in a wide variety of diseases.
- Seeking an answer to the problems with natural killer cell dysfunction in ME/CFS, the group looked for ways that calcium signaling – a key factor in NK cell cytotoxicity – might be impaired, and found it in disrupted TRPM3 functioning. Numerous small studies validated that finding in ME/CFS as well as Gulf War Illness and long COVID. Several studies suggested that the relief some people with ME/CFS get from low-dose naltrexone may lie in the drug’s ability to open up the ion channels again and restore calcium levels.
- In a recent paper “Potential pathophysiological role of the ion channel TRPM3 in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and the therapeutic effect of low-dose naltrexone”, Wirth and Lohn proposed that the ion channel dysfunction the Griffith team found probably extends far beyond the immune system.
- They showed how a disruption of these small but ubiquitous ion channels in the small nerve fibers and the brain could play a large role. Underactive TRPM3 ion channels could leave the blood vessels in a vasoconstricted, or tightened-down, state – a key theme in the Wirth’s original hypothesis regarding ME/CFS.
- TRPM3-induced nerve degeneration could contribute to “microcirculatory disturbances, radicular compression, reactive oxygen species, and autoantibodies”.
- In the brain inhibition of the feel-good chemical GABA could result in hypervigilance and elevated sympathetic nervous system activity (fight/flight). That energy mismatch (high energy demand/low energy levels) leads to cognitive problems, brain fog, and hypersensitivities to noise, light, and other sensory inputs.
- Because the GABA system also regulates muscle tone and tension, they propose that TRPM3 dysfunction may contribute to skeletal muscle pathophysiology, leading to muscle fatigue and post-exertional malaise (PEM).
- They asserted that “investigating TRPM3 dysfunction could be crucial for advancing our understanding of the pathophysiology of these conditions.”
- Wirth and Lohn do not mention possible TRPM3 enhancing treatments but one supplement and some drugs are a possibility. Pregnenolone is a supplement that both Dr. Teitelbaum and Dr. Myhill have recommended pregnenolone for people with ME/CFS. A precursor to estrogen, progesterone, testosterone, and cortisol, PS is called a “neurosteroid” – a steroid produced in the brain that modulates nervous system excitability. Dr. Myhill calls pregnenolone “the mother and grandmother of all steroid hormones”.
- Dr. Teitelbaum reported that he often finds low pregnenolone levels in ME/CFS, believes it may reflect an underlying infection, and has proposed a supplementation regimen to battle that. (See blog). Referring to the chronic stress ME/CFS patients’ bodies are under, Dr. Myhill wrote that “pregnenolone may be particularly pertinent in the treatment of chronic fatigue syndrome”. During chronic stress, the “pregnenolone steal” results in more stress hormones being produced at the cost of sex hormones such as testosterone, estrogen, and progesterone.
- Dr. Ronald Hoffman of “Intelligent Medicine” reports that PS is an endocannabinoid, akin to CBD (cannabidiol), and like CBD, can help with pain, mood, and improve memory. Gven its potential effects on hormones, he recommends that it be taken under the supervision of a health practitioner and that baseline blood levels of pregnenolone and its metabolites be assessed over time.
- A TRPM3-enhancing drug called nifedipine is used mostly to relax blood vessels and allow more blood flows in, and treat esophageal spasms. It appears to be a cousin to nimodipine which can be helpful in ME/CFS.
- Time will tell if Marshall-Gradisnuk, and Wirth and Lohn, are correct that dysfunctional TRPM3 ion channels are contributing to, or even causing, ME/CFS and similar diseases. The evidence for TRPM3 dysfunction in ME/CFS – quite a few positive small studies – is broad but shallow and bigger studies are needed.
- While eyes may glaze over at the advent of yet another new hypothesis, each one opens a new window and a new way of looking at these diseases. I remember thinking at one time that the field was stagnant and searching for new ideas. No more. With more eyes than ever taking a gander at these diseases, more and more creative ideas are emerging.
- Coming up – the hypocortisolemic ASIA hypothesis for ME/CFS.
Messed Up Sodium/Calcium Ion Channels?
Ion channels let ions like sodium, potassium, calcium, and chloride pass through the cellular membranes into the cell where they affect all sorts of cellular functions. The TRPM3 ion channels control the flow of sodium and calcium into the cells.
These ions are a big deal. Calcium plays a role in everything from energy production, muscle contraction, neurotransmitters, gene expression, and cell death. Sodium helps maintain the health of the cell membranes and plays a role in electrical signal generation, fluid balance, nutrient transport, and others.
Problems with these small channels have been implicated in a broad range of diseases including migraine, epileptic encephalopathies, chronic pain, low back pain, blood vessel functioning, cerebral palsy, eye diseases, and more. TRPM3 channelopathies can have such a wide reach because they’re found on sensory neurons, on smooth muscles in the blood vessels, brain and spinal cord, skin, pancreas, kidney, ovaries, pituitary gland, and the testes.
Griffith’s TRPM3 Gambit
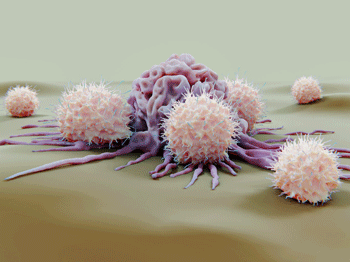
The TRPM3 saga began with trying to explain why natural killer cells (white, knobby cells) are not functioning well in ME/CFS.
Griffith’s TRP ME/CFS study was driven by numerous findings of NK cell dysfunction in ME/CFS. Seeking an answer to them, the group looked for ways that calcium signaling – a key factor in NK cell cytotoxicity – might be impaired, and found it. Increased levels of genetic polymorphisms in TRPM3 (and TRPM8) ion channels and acetylcholine receptors in ME/CFS suggested something had gone wrong. From there, the hunt was on.
The next two studies found reduced levels of TRPM3 ion channels on natural killer (down 50%) and B-cells, and an accompanying reduction in intracellular calcium in ME/CFS. Noting that the endogenous opioid system and TRPM3 receptors are involved in pain production, three lab studies found a new reason low-dose naltrexone might work when it does: naltrexone restored the TRPM3 ion channel activity in ME/CFS patients and re-established the appropriate Ca2+ signaling.
An overexpression of TRPM2 channels on NK cells and in TRPM7 ion channels pointed to more possible problems with calcium mobilization in ME/CFS. Recent studies also found dysfunctional TRPM3 ion channels in Gulf War Illness and long COVID.
While most of the Griffith studies have been very small, they have consistently pointed to TRPM3 ion channel dysfunction in ME/CFS and Gulf War Illness and long COVID.
TRPM3 Supersized?
With the recent publication of “Potential pathophysiological role of the ion channel TRPM3 in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and the therapeutic effect of low-dose naltrexone”, Wirth and Lohn, perhaps not surprisingly, joined the TRPM3 fray.
In 2016, Wirth and Scheibenbogen proposed intracellular calcium problems driven by problems with a sodium transporter played a core role in the energy production problems in ME/CFS. Since then, in a series of hypothesis papers, Wirth has greatly extended their original ideas. See below.
Wirth and Lohn started their present hypothesis paper on an expansive note, stating that it is “unlikely that ion channel dysfunction (in ME/CFS) is limited to a single organ or cell type…”, and asserting, “An isolated TRPM3 dysfunction in NK cells alone does not satisfactorily explain the pathophysiological role of TRPM3 in ME/CFS.”
They also pointed out how different diseases like ME/CFS may be. While increased TRPM3 activity and calcium levels have been associated with ME/CFS-like symptoms such as cognitive, and musculoskeletal problems, and pain – the TRPM3 problems in ME/CFS involve a “loss of function“; i.e. reduced TRPM3 activity and reduced calcium levels. Until ME/CFS popped up, no diseases had been associated with reduced TRPM3 functioning.
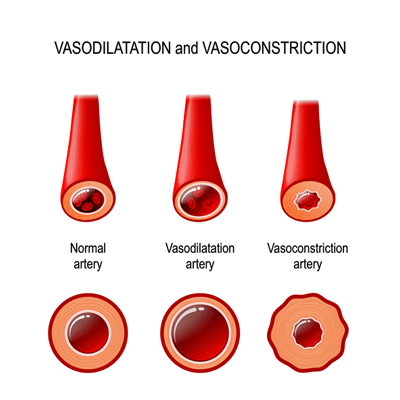
Wirth and Lohn propose dysfunctional TRPM3 channels may be vasoconstricting the blood vessels – thus preventing normal blood flows.
They explained how a disruption of these small but ubiquitous ion channels in the small nerve fibers and the brain could play a large role in ME/CFS. They believe that underactive TRPM3 ion channels may result in reduced CGRP levels, leaving the blood vessels in a vasoconstricted, or tightened-down, state – a key theme in the original hypothesis. Reduced CGRP levels result in sodium overload, increased intracellular calcium levels, and ultimately mitochondrial dysfunction.
Moving onto the small nerve fibers, they write, “it is difficult to believe that a functional defect of TRPM3 as found in natural killer cells… should not be finally involved in the degeneration of the small nerve fibers”, and then proposed that the TRPM3-induced nerve degeneration may contribute to “microcirculatory disturbances, radicular compression, reactive oxygen species, and autoantibodies.”
In the brain, they propose underactive TRPM3 channels may be inhibiting the production of the feel-good chemical GABA. Since GABA inhibits the excitatory neurotransmitter glutamate, reduced GABA activity could result in the hypervigilance and elevated sympathetic nervous system activity (fight/flight) found in ME/CFS.
They noted that the brain overstimulation that’s probably present exerts a double whammy by taking place in a brain that’s already deprived of normal amounts of blood (i.e. energy). That energy mismatch (high energy demand/low energy levels) leads to cognitive problems, brain fog, and hypersensitivities to noise, light, and other sensory inputs.
Because the GABA system also regulates muscle tone and tension, they propose that TRPM3 dysfunction may contribute to skeletal muscle pathophysiology, leading to muscle fatigue and post-exertional malaise (PEM).
Furthermore, the increased blood pressure, heart, and skeletal muscle tone produced by mental stress results in more energy consumption, putting more stress on a broken energy production system, causing more calcium overload, and impairing blood flow to the muscles, leading to skeletal muscle damage and mitochondrial dysfunction. That’s an interesting idea given the muscle pain I often associate with mental exertion.
Overall, they propose that “TRPM3 dysfunction has significant pathophysiological potential, contributing to malperfusion, mitochondrial dysfunction, and small fiber degeneration in ME/CFS”.
They asserted that “investigating TRPM3 dysfunction could be crucial for advancing our understanding of the pathophysiology of these conditions.”
Treatments? TRPM3 Agonists (Enhancers)
The article does not discuss treatments, but some TRPM3-enhancing drugs have been developed, and a recent review called TRPM3 “a new kid on the block in pain treatment?“.
The Pregnenolene Possibility
The best-known source – pregnenolone sulfate (PS) – is a supplement. A precursor to estrogen, progesterone, testosterone, and cortisol, PS is called a “neurosteroid” – a steroid produced in the brain that modulates nervous system excitability. Dr. Myhill calls pregnenolone “the mother and grandmother of all steroid hormones”.
Low pregnenolone levels have been found in ME/CFS.
Dr. Teitellbaum reported that he commonly finds dramatically low pregnenolone levels in people with ME/CFS, which he believes are a sign of infection. While he stated that he found pregnenolone can help, he reported that taking it in combination with other supplements can be more effective.
In 2011, he recommended that ME/CFS patients who are not taking statins and have low or low-normal pregnenolone or low cholesterol levels add CoQ10 (200-400 mg a day), pregnenolone (100-200 mg a day), and omega fish oil for three months. If your illness had a flu-like onset, he recommended taking zinc 25 mg a day for 3 months, then 15 mg daily from then on, and possibly adding a statin drug for a couple of months.
Referring to the chronic stress ME/CFS patients’ bodies are under, Dr. Myhill wrote that “pregnenolone may be particularly pertinent in the treatment of chronic fatigue syndrome”. During chronic stress, the “pregnenolone steal” results in more stress hormones being produced at the cost of sex hormones such as testosterone, estrogen, and progesterone. Adding in more pregnenolone helps to rebalance the stress and sex hormones. Myhill uses an Adrenal Stress Profile test to determine if a deficiency of cortisol is present, and if so, uses pregnenolone in 50mg capsules that she suggests absorbing under the tongue.
Dr. Ronald Hoffman of “Intelligent Medicine” reports that PS is an endocannabinoid, akin to CBD (cannabidiol), and like CBD, can help with pain, mood, and improve memory. He states that PS was briefly used as an anti-inflammatory in the fifties, but its poor bioavailability led to it being abandoned. Worried about its effects on DHEA and other hormones, he used it in small amounts in his patients until two studies using 500 mg/day found that it helped with low back pain and depression. He wrote that PS is now being studied in multiple sclerosis to dampen neuroinflammation.
Still, given its potential effects on hormones, he recommends that it be taken under the supervision of a health practitioner and that baseline blood levels of pregnenolone and its metabolites be assessed over time. His Bottom Line is:
“Pregnenolone’s wide range of benefits, its relative freedom from side effects, the reassuring fact that it’s one of the body’s most prevalent natural hormones, and its ubiquity as an over-the-counter supplement warrant its investigation for a wide range of ailments for which there exist few safe and effective options”.
Drugs
A TRPM3-enhancing drug called nifedipine is used mostly to relax blood vessels and allow more blood flows in, and treat esophageal spasms. It appears to be a cousin to nimodipine which can be helpful in ME/CFS.
Perhaps the most potent TRPM3 enhancer – a drug called CIM0216 – is available only for research purposes. On a longer timeframe, there’s the drug Wirth and company are attempting to bring to market to treat ME/CFS.
Conclusion
Time will tell if Marshall-Gradisnuk, and Wirth and Lohn, are correct that dysfunctional TRPM3 ion channels are contributing to, or even causing, ME/CFS and similar diseases. The evidence for TRPM3 dysfunction in ME/CFS – quite a few positive small studies – is broad but shallow and bigger studies are needed. Still, it is good to see two different groups – the Griffith group with its focus on immune cells, and the Wirth and Lohn with their focus on blood vessel functioning and energy production – mesh.
While eyes may glaze over at the advent of yet another new hypothesis, each one opens a new window and a new way of looking at these diseases. I remember thinking at one time that the field was stagnant and searching for new ideas. No more. With more eyes than ever taking a gander at these diseases, more and more creative ideas are emerging.
- Coming up – a review of an ME/CFS/long-COVID hypothesis focused on infection/vaccines, inflammation, and, importantly, the HPA axis – the hypocortisolemic ASIA hypothesis
The Wirth Series of ME/CFS Hypothesis Papers
- Hypothesis Predicts Major Failure Point in ME/CFS
- The Blood Vessel Crunch: A Unifying Hypothesis for ME/CFS
- No Neuroinflammation Needed? An Epic ME/CFS Hypothesis Series Winds Up
- ME/CFS, MCAS and Gynecological Disorders – Klaus Wirth’s Blood Vessel Diseases
- Could the Blood Vessels…That Feed the Blood Vessels- Be Ground Zero for Long COVID and ME/CFS?
Please Support Health Rising and Keep the Information Flowing






Oh my God, yes, it’s ion channel stuff, especially sure of that with viral onset. That’s why brain retraining can work but it’s a temporary fix sometimes. It’s also why deeper into the disease, people like me, we have trouble near salt sea air and do better away from it. Oh, the autoimmune issues are profound. The viruses attach to immune cells and screw up what they consider abnormal. What a mess! In advanced ME, I’ve now got autoimmune MCAS, diagnosed PID, and now diagnosed with Bullous Pemphigoid and FT Dementia, the real deal, at age 61. I weigh 105lbs, pots etc. Thank you so much for your work. Christine
But why are the ion channels and Natural killer cells not working properly? Low NADH, like the rest of the body, due to mitochondrial disfunction? In at least a subset of patients it’s probably Epstein Barr, and the proteins it produces. Currently the only treatment proposed for calcium ion channel problems is Low dose Naltrexone……….which only helps some.
Heavy metals are notorious for suppressing NK cells. There are many ways to increase NK cells naturally.
Exactly. Low NADPH comes up everywhere in my research with my symptoms. Even taking vitamin K will put me on oxygen for a few hours because it will eat up the glutathione. We need that to stop the excessive ROS. We have barely touched on the 400 genetic variations of G6PD deficiency. It had been found even in the brains of Parkinson’s.
Hi Cort.
I’m having trouble digesting the idea that TRPM3 channel dysfunction cam lead to CFS.
This is because I am not reading in the blog why it is dysfunctioning– what has affected– or neglected– the channel (s)
The commonality that I found between skeletal muscle, cognitive cells and limbic system cells is that all 3 seem interlinked (you think hard and you get muscle pain…and my friend can’t follow my logical train of thought and is close to tears.
They all depend on a7 Nicotinic Acetylcholine receptors. Not on other nicotinic ones, and not on any of the many muscarinic ones. Not only is there hardly any other system in the body usimg this a7 Nic (maybe one other) but also, those areas do not use any muscarinic receptors.The CNS is reported to use the a7 Nic widely (so we will add nerves/neurons)
The only other things I can find that also use a7 Nic are the vessel Endothelial cells, T and B white cells, pre-limbic cortex. Please forgive any omissions.
But the Endothelial cells respond to ACh oppositely to the manner of skeletal muscles and cognitive cells: they create Nitric Oxide to make the smooth muscle cells of the vessels/arteries RELAX. Our skeletal muscles, cognitive cells and pre-limbic cells need Long-term Potentiation in order to lift something or to remember something. To flex muscles or to make the mind work.
I just read that in deep sleep (delta) the a7 receptors in the prefrontal (cognitive) cortex are inactive. But you can bet that they are active in REM sleep when memories are “consolidated”.
What I do agree with is that the ACh triggrrs opening of what I believe is this TRPM3 to allow sodium in and then attract potassium in.
And I agree that the muscles in CFS are left, after exertion, with excess sodium. But the reason there is that the muscle cells have turned anerobic and that renders only about 8% of the ATP units that aerobic renders. And, yes, there is an ROS overload. Without enough ATP, few things can be transported in or out through the TRPM3s. Calcium too becomes excessive and too much triggers cell death via PAD enzyme.
Hmmm… this could be why T-cells are overworked.
They vascular endothelium fails in its blood pressure role, using a7 Nic receptors to trigger dilation,
all of the above takes place because of hypoperfusion, Ischemic Cascade occurs, and now the T-cells come to clean up.
My point us not that TRPM3 channels are actually passing sufficient quantities of atoms– they are not. But I dont blame them. I blame insufficient a7 Nic receptor activity.
That suggests one of:
a) damage
b) lack of ACh
c) too much Acetylcholinesterase
d) same as c) except because AChEsterase receptors ate damaged or underexpressed.
On that note… could the a7 Nic receptors be underexpressed?
My theories include that the brain may be too hot and forbids the Pituitary to send much Thyroid hormone to the mucles since that would generate more heat. Is it true that CFS patients often feel cold– yet do not shiver?
Could you explain why sea salt air is a problem?
I would also like to know too, my country is one big coastline. Everytime i used to be near the sea I would get worse. All my family would be on the beach and i would be in bed with flu-ish symptoms.
Interesting you mentioned Bullous Pemphigoid. The whole time I was reading/listening I was seeing possible connections to a rare genetic skin disease I have, Hailey-Hailey Disease ATP2C1 gene that encodes calcium pump. My HHD wounds got significantly worse as I started having CFS symptoms of weakness in legs, brain fatigue etc etc.
And LDN is successful in helping many with HHD.
If part of the pathology discussed above is low CGRP then should we be asking what happens to patients like me who go on anti CGRP migraine drugs, who also gave an ME clinical picture? I know that I’m not the only one out there who took or takes anti CGRPs
I takw anti-CGRPs and they have dramatically improved my sensory sensivity and migraine. Which is why I feel a bit off about this theory. That said I strongly believe there is not 1 cause of ME. There is just way too much variation in drug response for it to be a single pathology.
Right! I found that part of the hypothesis curious. Of course, who CGRP plays a role in is unclear, but some people with these diseases do well on anti-CGRP drugs.
Im taking CRGP antagonists for migraine…grapeseed extract, butterbur and ginger. Have not noticed any me cfs downside.
Thanks Cort and Geoff – really interesting!
I wonder how POTS and hEDS-type symptoms fit with the vasoconstriction mentioned in this theory, as I thought there were issues with the blood vessels perhaps not being able to constrict properly to pump blood back up from the lower limbs (hence the vasoconstrictor midodrine being used in POTS)?
I have personally found LDN very useful – I’m severe and an hour or two after I take it it’s like I’ve moved up a level and am less foggy and more capable both physically and mentally. But when it wears off then it’s gone again – definitely something that helps but unfortunately for me hasn’t been a cure.
Hi Chips. I saw a talk Wirth gave at the 2023 Charite Berlin ME/CFS conference. He said the vasoconsriction is happening in the blood vessels in the muscles and brain (resistance blood vessels) but that in the blood vessels leading back up to the heart centrally, the problem is one of excess vasodilation (and the orthostatic intolerance that comes with it). In that talk, he said there is currently no joke to treat these two problems simultaneously.
Hi Patrick! Oh wow, that’s a conundrum and a half isn’t it – I wonder why both occur together and if this happens in any other illnesses. Thanks for letting me know, I can feel myself falling into a google rabbit hole on this already…!
You are welcome Chips! It is indeed a conundrum. The alpha-1 receptors in the bigger blood vessels up to the heart are often desensitised through stress or blocked through autoantibodies – this causes a lack of vasoconstriction. Meanwhile the beta 2 and muscarinic 3 receptors in the skeletal muscle are also desensitised through stress and blocked by autoantibodies which – in this case – cause a lack of vasodilation.
But then, you wouldn’t expect anything about ME/CFS to be straightforward, right? 😉
In that talk Klaus said ME/CFS patients should not take a vasodilator – they’ll make one problem better and another problem much worse.
His and Scheibenbogen’s paper ‘Unifying Hypothesis on Pathophysiology of ME/CFS’ is well worth a read on all of this.
Does anyone know how topiramate would affect pwME or LC?
I read that Topiramate:
• inhibits voltage-gated Na+ channels
• inhibits high-voltage activated Ca2+ channels
• antagonises non-NMDA-type glutamate receptors
• potentiates GABA receptor activity
• potentiates carbonic anhydrase enzymes
… I can see it would likely have some effect because the same ion channels are involved but I don’t understand if it would be bad for us to take?
I ask because it’s so widely used for migraine – and in my own case I took this for years prior to my ME onset, so I’m interested if this could’ve contributed…
Thanks Cort. I currently use salt water supplementation, and add salt to food, as the normally recommended means to combat my quite severe orthostatic intolerance – perhaps my most disabling symptom. This is to constrict blood vessels, which supposedly helps force blood upwards towards the brain and thus prevent faintness while upright. It seems to help a bit.
But here, if I understand correctly, we have the suggestion that we may need something to DILATE the blood vessels in order to promote blood flow to the brain.
So how should we reconcile these two apparent opposite approaches to improving blood flow – should we be constricting or dilating the blood vessels in order to improve perfusion to alleviate OI and improve brain function and thus all the body systems that in turn affects?
I hope this Q makes sense, given that I struggle quite a bit with cognitive processing and my memory of what I’ve just read. <3
Elizabeth, I do not mean to answer instead of Cort.
If the muscle cells end up hoarding sodium as a result of failed exertion,
or alternately,
if the kidneys are damaged by the disease that triggered CFS and do not sufficiently retain sodium,
then drinking salt water will help to restore your blood volume. Both water and salt are electrolytes and if one is lost, the other must be excreted as well.
In long covid the kidneys usually have been damaged. This is because smooth muscle is damaged and that type of muscle is found in the blood vessels and in the kidney microvessels– and most or all other organs.
A number of readers of this website have reportec having low blood volume.
It might be problematic to ingest only salt without any water– but in your case you are ingesting a balance. So you are helping… not hindering. Equally, if you indeed would otherwise have low blood volume, and then you were to drink only water, without salt, the water would not be retained as much.
Also, Elizabeth,
the issue is not only whether vessels are dilated as they enter the head —
there needs to be a balance of constriction and dilation in the whole body when one area needs more blood and oxygen.
The legs need more to stand you up, but the brain needs more also to balance you. That means the various organs other than the heart should get less blood temporarily.
Perfusion is achieved by dilation in the area needing perfusion. But some other areas must be constricted to free up extra blood and to help to pump blood back to the heart for your brain to receive.
And you can imagine how too little blood volume would make the system less effective.
What you truly need to improve blood pressure regulation is sufficient nitric oxide to dilate the vessels for perfusion.
This is a job for a doctor, or doctor of functional medicine.
It has come to light that taking L-arginine supplements provide the substance that gets converted to Nitric Oxide (NO). L-Citrulline comes from watermelon and not only skips a chemical step, being more quickly converted to NO, but also more NO is made in the process.
You might even be interested in the work and supplements of Doctor Steven R. Gundry who has been a heart surgeon all of his life. He wants now to prevent heart disease. NO production naturally is reduced as we age.
desgod306@yahoo.com
Christopher,
I’m no doctor, but; I am a patient.
I have been supplementing with L-Arginine + L-Citrulline for years.
Both work for me. 👍
They may not work for others. 🤷♂️
Understanding what cofactors to take with these two amino acids is also important. 🔑
Both also compete with other amino acids for absorption- particularly L-Lysine.
I wish you luck on your healing journey. 🍀
Thank you so much for sharing. 🙏
Christopher, What brands and amounts of L-Arginine + L-Citrulline do you use? And what do they help with?
Tracey,
I use the NOW brand for both L Citrulline + L- Arginine- free form- powder format.
I find the taste of L-Arginine hard to tolerate in powder format, however; powder provides me with optimal absorption results. 👅
Both of these amino acids act as vasodilators (causes blood vessels to widen). 🚢
This improves my circulation. 🩸
More oxygen and nutrients reach the cellular level. This helps with mitochondrial fuel efficiency. ⛽️
My doctors have noticed, via CBC biomarkers, every 90 days, that my blood pressure and my blood sugar levels have returned to within normal ranges (thresholds). 🧫
From an antidotal perspective, I feel like air is being released from a balloon. 🎈
Another analogy would be as if too much PSI pressure is being released from a tire. 🛞
It feels as if more blood is reaching small fiber areas in my body.
This also helps diminish a little muscle fatigue and pain I experience.
The Nitrous Oxide relaxes the inner walls of my muscles so well that this provides me with a temporary calm yet slightly synergistic energy.
There is definitely some sort of somatic- neuronal arousal I experience.
It wears off though in a few hours.
Don’t use this product if you are susceptible to any type of herpes reactivation- : to include EBV (Epstein Barr), Varicella (shingles), HSV6, and/or HSV 1 or 2.
Some scientific experiments have indicated that L-Arginine can cause dormant 🦠 to reactivate. ⚠️
If you are a patient taking any sort of anti-viral medication 💊- these medications will compete with the amino acids aforementioned for absorption/efficacy.
I hope this data helps you in some way. 🤞
Best of luck to you. 🙏
Thank you Christopher. I was ready to order until I read about the possible herpes reactivation, a key problem of mine. Bummer!
This is good news.
One of the CFS ptoblems consistently found is insulin resistance. You have reported your blood sugar balancing out.
Only in the past year or two has a researcher addressed in a very public way what leads to insulin resistance and further to diabetes T2.
And it is now being broadcast that diabetes can be reversed.
1. We spike our blood sugar often with high carbs or high sugar, BEFORE we eat lower glycemic index foods.
2. There is a INSULIN spike to compensate, but we are not aware of either spike.
3. As this progresses through the years, the resting insulin level gets gradually higher and higher– to protect us.
4. The cell does not want EXTRA glucose and develops resistance or insensitivity to INSULIN.
Then one day the patient notices a problem, and now 10-20 years after the process began, only now is the resting insulin tested.
5. One of the best strategies to reverse this is to eat “savoury” before sweet in the morning– and at any time.
Things that are slower to digest, such that it also slows down the release of any sugars into the blood, and makes it more paced.
Christopher,
Thank you for your quantitative + qualitative feedback. 😊
I have cut out refined sugar entirely. 💯
I limit my carbohydrate intake.
My diet consists primarily of protein and fat. 🚫
I no longer consume any processed foods.
I have initiated cell autophagy via intermittent fasting.
I have initiated regenerative neuro plasticity.
I changed my metabolic fuel source from sugar to fat. ⛽️
I detoxify with antioxidants.
I have modified my sleep cycle. 💤
I supplement with specific amino acids, vitamins, and minerals throughout the day that target specific organs (brain, liver, muscle, connective tissue, thyroid, lungs, heart, stomach, kidneys, GI tract, and of course circulation). 🎯
Exercise is still a problem. ⚠️
But, I’m working on this. 🧩
I wish you the very best in your journey to heal and recover. 🙏
Christopher, could you offer a few more specifics regarding the use of L-Arginine + L-Citrulline ? What are you addressing by taking these? Is there a significant improvement of whatever is being addressed and in what way? And any other information else you might find useful.
Thanks.
Lise,
Please see my response to Tracey. 🙏
I think you will find the specifics and the details you are looking for in my reply to her. 🤞
I wish you well on your journey to recovery. ❤️🩹
I’ve never been able to get the vasodilation/vasoconstriction thing straight but Patrick reported that
” I saw a talk Wirth gave at the 2023 Charite Berlin ME/CFS conference. He said the vasoconsriction is happening in the blood vessels in the muscles and brain (resistance blood vessels) but that in the blood vessels leading back up to the heart centrally, the problem is one of excess vasodilation (and the orthostatic intolerance that comes with it). In that talk, he said there is currently no joke to treat these two problems simultaneously.”
I need to correct a couple of points made by the “other” Christopher.
The L-arginine is converted to
not Nitrous (laughing gas)
but
Nitric Oxide by the body, and it will not do it by force, just because it finds arginine… it will do it as needed by vessels.
L-Citrulline can also be converted to NO.
This is only my opinion:
(supplying substrate from which NO can be made will not in itself cause the vessels leading back to the heart to further dilate. It is a “decision” made by the endothelial cell to relax the smooth muscle cells with NO)
The way I see it is that if there is faulty NO system function owing to massive remodelling (loss of and substitution for endothelial cells during illness),
then the supplement can somewhat bypass the problem. Bur you are not dumping NO into your vessels.
Contrary to what Christopher (no ‘D’) said, the citrulline and arginine are not vasosilators. They are precursors. This gives me hope, though. He is seeing restoration of NO function. The body hopefully can repair itself, right?
But you need to deduce the intervention needed in YOUR body– the boost that the tired cells need.
We need to find out what is broken in the endothelial cells. Are the numbers too low?
I can say decisively that a7 Nicotinic receptors on Endothelial cells act as baroreceptors, sensing increases and decreases in heart pumping pressure against the walls if vessel. They need to very quickly react by converting the two L’s that we are discussing into vasodilating NO to *accomodate the increased blood volume.
But they will not do so if their region is not the region Exerting. OR– if they “think” that they ought not to do so. Eg. if head office (hypothalamus) is enforcing hypothermia and is not supplying muscle cells with thyroid hormone in order to NOT allow the brain to overheat.
We have the added problem of being stuck, possibly, in fight/flight mode
and that is about vagus nerve tone.
As I mentioned once before, a simple way to switch your vagus nerve on, or switch to rest/digest mode is to breathe more deeply by expanding your stomach area downward, which creates a vacuum on your diaphragm, drawing it down, which effectively preloads MORE oxygenated blood into the heart.
As I understand your blogs, Cort, the preload can tend to be too low if the body does not go properly into rest/digest wgen you need it to.
By the way, does anyone here experience blood pooling in the legs/feet? If so, is this worse during sleep, or is it somewhat alleviated by sleep?
Regarding the recommendation of pregnenolone for ME/CFS, since it converts to various sex hormones, I was concerned, having had breast cancer, if it would safe to take. So, the answer is ‘it’s complicated.’
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8568650/
The safety depends on a lot of things and if you are a post menopausal women with a uterus, pregnenolone MAY be protective of endometrial cancer but also MAY put you at greater risk for ovarian cancer. There are a lot of moving parts here; like your own genetics and what hormonal levels you already have, you age, your weight and reproductive status.
Good point. I am a stage 3 ovarian/uterine cancer survivor. My doctor put me on pregnenolone, which seemed to preferentially convert to too much estrogen, not a good thing…
I did have better luck with taking DHEA and hydrocortisone. Everyone’s hormone situation is different. I found a DUTCH Complete test worked well to balance my hormones.
Pregnenolone is my enemy. Twenty years ago, and already eighteen years into my ME/CFS saga, a CFS doctor trialled me on it. Instead of helping, it sent me into a massive crash from which I eventually only recovered to a small degree, nowhere near how I had been previously (which had been bad enough, as I couldn’t work and could only get out of the house a couple of days a week). I have no recollection of the dose, and the doctor and his clinic no longer exist. Needless to say I didn’t risk trialling anything else for many years thereafter.
That really said something about your physiology! Did the doctor have no idea why that big crash occurred?
Cort, he said he’d never had it happen to anyone else and had no clue why I responded like that. He was anxious to experiment with other drugs to try to reverse the damage, but I was not prepared to be a guinea pig after that. In any case I was no longer able to travel to his clinic, so could only speak to him on the phone. Prior to that, it hadn’t even crossed my mind that an experimental treatment might make me worse! I’d tried any and every treatment over the previous 18 years – nothing had helped but nothing had made me worse.
I have been advocating since 1997 for the recognition and proper diagnosis of my condition, which involves low potassium levels (hypokalemia) and an imbalance in the aldosterone-to-renin ratio. Despite my efforts, success has been elusive. This is a complex endocrinological issue, but a simple cortisol/ACTH test could serve as a crucial starting point, potentially leading to significant symptom improvement. My own journey was long and challenging, ultimately resulting in a diagnosis of Addison’s disease and Conn’s syndrome.
https://swaresearch.blogspot.com/2024/08/when-aldosterone-to-renin-ratio-arr-is.html
Please tell me in which direction your aldosterone-renin ratio was wrong:
too little aldosterone, or….
Renin: 2.3
ACTH: 5.3 pg/ml (slightly below normal).
Aldosteron: 56.5 pg/ml (within normal range).
Aldost.Ren.Quo.Index: 24.15+ (above the normal range, which might indicate an issue with aldosterone-renin balance).
Blutzucker nü: 74 mg/dl (within normal range).
Cortisol:
0 ug/dl: 4.7 ug/dl (slightly below normal).
30 ug/dl: 11.3 ug/dl (above the expected range of 0).
60 ug/dl: 13.7 ug/dl (above the expected range of 0).
Kalium: 3.61 mmol/l (on the lower edge of normal).
Natrium: 140 mmol/l (within normal range).
Renin: 2.3 pg/ml (slightly below normal).
Lifelong low blood pressure, low energy and muscle weakness my Cortisol until 2007 was 1.1.
Reference: S.E.D. Medical Laboratories Lanu Stoddart, M.D., Med. Dir. 5601 Office Blvd., NE Albuquerque, New Mexico, 87109 505/727-6300 800/999-LABS Central Lab CLIA #3200534750
CLIENT NAME / ADDRESS Physicians Group 5400 Gibson SE Albuquerque, NM 87108
REQUESTING PHYSICIAN EDWARDS, TERRY H. MD
New: Persistent Autonomic and Immunologic Abnormalities in Neurologic Post-Acute Sequelae of SARS-CoV2 Infection
https://www.neurology.org/doi/10.1212/WNL.0000000000209742
I have a question. Do they have any conclusion why the ion channels get disturbed, causes for the problems?
And another, maybe not just on this article, but why is there a delay of PEM? My memory tricks me 😉 Why do I get worse in the afternoon the day after my exertion? And not immidiately? Is it about the same as you get sore muscles a day or two after weight lifting (healthy persons)?
I don’t know if either paper took a stab at why they are damaged. In the end the putative answer for so many things often seems to be an attack by the immune system (inflammation/autoimmunity) but I don’t know.
It’s not the same as sore muscles because with the case of sore muscles its basically just sore muscles – not all the other stuff fatigue, bodywide pain, sleep problems, etc.
In my experience the soreness is different. Right now some studies are pointing to problems with muscle repair mechanisms – they don’t seem to be clocking in like they should.
In fact, at the molecular level, ME/CFS patients cells don’t seem to understand that exercise is going on.
Sorry, I expressed myself badly about sore muscles. I ment that both PEM and sore muscles after training comes delayed. I’m well aware that they are different things. I didn’t mean muscle soreness from PEM.
My question really is why PEM is delayed?
Those of us with PEM all understand your question, Kajsa, but I feel like most of us don’t have confidence in an explanation. Maybe because we aren’t recovering, the very simple acts we perform 24 hours later are being performed–unbeknownst to us–when we are totally depleted. And so it is not really a delayed reaction, it is just a reaction to something we just did that we didn’t think was exertional.
Possibly the lymphatic system becomes overloaded with metabolic waste from exercise?
I have used LDN several times over the last 4 years. The first time was great. Much improvement in stamina & reduction in bad reactions to chemicals but it lost its effectiveness after two weeks suffering from a virus, which never showed up as COVID when testing. I’ve justed started again after a break of a few months. Hoping for good results again.
My query is, would larger doses of naltrexone work better? I couldn’t see or understand dosage on the chart illustrations.
This is interesting:
https://www.medrxiv.org/content/10.1101/2024.08.14.24311980v2?fbclid=IwY2xjawEyc0BleHRuA2FlbQIxMQABHYb_tT2wzCtzXhyaFeKXGMx9eu5AiiABfZfysf3QSIgKV9oOlpTQwEE0IA_aem_JgBHZ0VIxA34LQMJSCcGbA
It is very good that you gave us this link.
“These findings suggest a mechanism linking systemic immune dysregulation to muscle-specific mitochondrial dysfunction in PASC.”
…in Post-Acute Sequelae of Covid.
As you know, a flu will cause a fever to make various cell functions impossible or at least weaker. The Hypothalamus (head office) sets a temporary new body temperature set point.
This should go back to normal after antibodies kill all of the virus.
But we all know on Healthrising now that some cells renain stuck in the innate immune response and never reach healing. Why? is of course the main question.
But notice that you don’t remain in a fever state! Yet you DO seem to remain in an inflammation state.
Do any of you have below-comfortable body temperatures? Being below 97 F by 1–1.5 or 2 degrees is classified as
hypothermia. You don’t have to be buried on a ski hill or dumped in mid ocean to be hypothermic.
There is a Sepsis association in the USA which declared that “long-covid is sepsis”
Sepsis is the body’s response to yoo much toxicity. Cytokine storm is too much toxicity. I believe that there is also a cytokine storm in pandemic-grade Influenzas.
My bottom line is that I believe many CFS patients nay be stuck in a hypothermia resulting from Sepsis.
Hypothermia too lowers cell function.
If you find yourself now asking the question ‘what can I do if I am hypothermic from sepsis’
my answer would be intensive anti-inflammatory nutrition, but not drugs.
Curcumin from turmeric is one of the most powerful. But there are many.
Also, antioxdiants are necessary. Cort has explained that reaction oxygen species ROS are in overload in CFS.
There is a good reason for that: anaerobic ATP creation has far more ROS byproducts and CFS patients who exert themselves switch quickly from aerobic to anaerobic.
So the very least you should do is supply your blood with many antioxidants.
And there us a fine line between
antioxidant
and
anti-inflammatory
In fact, when it comes to foods…. IS there any difference?
Cirus Bioflavonoids lower inflammation in blood vessels– and they are known antioxidants.
Thank you Cort,
If indeed this channelopathy was “causing” ME/CFS etc, the hypothesis needed to answer two questions:
a) how would it explain the widely fluctuating clinical picture with sudden dysfunction after stressful events? Does the channel work rather okay sometimes and then stop working suddenly? In my opinion, any hypothesis on ME/CFS should start with explaining PEM – after all, this is what defines ME/CFS …
b) this channel is supposed to also cause (or play a role in) GWI (and, possibly in FM, after all, LDN is supposed to work there too…). But GWI is not ME/CFS, at least not clinically. (It seems, for instance, that GWI does not go along with PEM, at least not like in ME/CFS: https://pubmed.ncbi.nlm.nih.gov/38777281/ ) Could the same channelopathy be at the root of two different diseases?
I doubt it. I´d rather not be surprised if in these diseases marked by profound oxidative, adrenergic, inflammatory etc stress quite a few membrane channels do not work properly.
Also, I think the putative “proof” (or at least support) for this hypothesis (the presumed clinical response to LDN) is a rather weak one as there es really no support for this claim on at least moderate evidence level.
I thought Memantine helped regulate both Calcium channel dysfunction and Glutamate excitotoxicity, both involved in CDR. Always wanted to try LDN but thought it was contraindicated with Mold/Candida issues.
Well, pregnenolone did not cure me (at least at the normal doses I used). What is PS in the Gist, please?
Pregnenolone sulfate
P.S. a small correction: name of Wirth’s co-author is Löhn, not Lohn https://pubmed.ncbi.nlm.nih.gov/38970055/ .
I suppose this should be him: https://www.youtube.com/watch?v=Se78x4-skbg and you can hear the pronunciation right at the start of the video.
Im confused when reading Ca overload in one instance but low intracellular Ca in another?
Thank you for this post & your wider explanation of ion channelopathy hypothesis. Just wanted to share my personal experience re treatment for sodium channelopathy – in 2011 from doctor clinician/researcher in Melbourne, Australia specializing in ME/CFS, & a very early advocate re role of gut biome (decades before gut bacteria became mainstream) & ion channelopathy in ME/CFS. Was prescribed low dose lamotrigine for sodium channelopathy to which – can only say – I had an explosive physiological response to – the effects of & repercussions I continue to live with 13 years on. Within three hours of taking, one lamotrigine 12 mg. dose produced severe Cerebral Palsy type symptoms – including 80% loss of vision lasting for one day. The overall Cerebral Palsy type response was acute for three days, with symptoms dampening down over a few years to, eventually, lie latent but then flare when provoked by environmental/emotional stressors. Though the flares now come less often & are, mostly, less severe, the Cerebral Palsy type symptoms – plus Tourette’s like symptoms – remain with me 13 years on. I lay no blame on the prescribing doctor. This is simply an outcome that couldn’t have been anticipated in an ME/CFS field still in exploration stage.
That’s really interesting. I’m probably generalizing too much because I don’t understand these drugs, but Lamotrigine is supposed to be a sodium channel blocker and can also block some calcium channels. It’s an anticonvulsant.
If I’m getting it right, the low functioning TRPM2 in ME would be causing low intracellular calcium and also decreasing the flow of sodium. So Lamotrigine might make both of those situations worse.
The channelopathies being looked at in sepsis are partly because of the loss of peripheral nerve function that can happen. Have they been looking at TRPM3?
The drug (fampridine) used in MS that sometimes increases energy, muscle use, and other functioning, is a potassium channel blocker and increases cystosolic calcium/potentiates calcium channel currents. It’s a convulsant. I wonder if it affects sodium channels?
Thank you for your reply – what you say is very interesting & offers explanation for the massive & life altering response I experienced after taking a single low dose of lamotrigine. My disappointment at the time with prescribing doctor was that he stepped back into defensive medicine mode rather than using my reaction as a guide to improve understanding of disease process & direction to appropriate treatment.
“Simply put, there is no known adrenal pool of pregnenolone for one cell to steal away from another, and no known mechanism has been described that could facilitate the transfer of pregnenolone between the mitochondria of different cells (in this case, from the mitochondria of cells within the zona reticularis to those within the zona fasciculata). Unfortunately, the most common figures used to teach steroidogenesis show a common pathway and typically do not specify the differential regulation of available enzymes between different steroidogenic tissues. This leads many to incorrectly assume there is a single “pool” of pregnenolone available for all steroid hormone synthesis within the adrenal.”
However preg might be working, there’s no such thing as a pregnenolone or progesterone “steal”.
https://www.zrtlab.com/blog/archive/reassessing-pregnenolone-steal/
Been reading about how trauma affects our immune system , nervous system and body. Some war veterans suffering from post traumatic stress syndrome show same symptoms as ME patients. Perhaps research could be done on how many have experienced something traumatic in their lives before they developed ME. After I caught a virus in 1989, I could not cry or smile for years . It was like being in deep shock. In England recently, the medical Journal says that perhaps talking therapy could help ME sufferers. When you hide feelings, stress hormones can lead to muscle, bowel and headache problems.
Many ME sufferers feel isolated. If you touch others, open up and communicate , the immune system improves. T cells spring into action. Doctors in America working with patients who have had a trauma in the past are working with eye movement treatment. Mindfulness helps too.
Perhaps their research will find a cure for ME if they can find a cure for Post Traumatic stress disorder too. What do you think???