

Geoff’s Narrations
The GIST
The Blog
The GIST
- Avindra Nath has proposed that B-cell exhaustion is driving ME/CFS, but in “Hypocortisolemic ASIA: a vaccine- and chronic infection-induced syndrome behind the origin of long COVID and myalgic encephalomyelitis“, Spanish researchers propose another suspect – exhausted T-cells. Both researchers start with an infection, identify an immune weakness that leads to exhausted immune cells – end up with chronic fatigue syndrome (ME/CFS).
- While Nath ends up in the temporal-parietal junction (TPJ) in the brain, these researchers end up at the pituitary gland in the brain: two immune hypotheses – two brain endpoints…Interesting!
- The pituitary gland is part of the HPA axis – one of two major stress response systems in the body. It produces adrenocorticotropic hormone (ACTH) which stimulates the adrenal glands to produce cortisol. In hypopituitarism – the pituitary problem these researchers believe is present in ME/CFS, the pituitary gland doesn’t produce enough ACTH – resulting in low cortisol levels and probably fatigue, inflammation, etc.
- The paper starts with the recognition that herpes and other viruses can inhibit HPA axis activity, that the pituitary gland’s location makes it particularly vulnerable to infections, and that depending on which way the HPA axis bends, either immunosuppression (high cortisol), or inflammation, or autoimmune diseases (low cortisol) may result.
- It turns out EBV may, in some individuals, target specific cells in the pituitary that regulate ACTH secretion. A direct EBV infection of these pituitary cells could decrease ACTH production and thus produce low cortisol levels.
- One ME/CFS study found anti-pituitary/hypothalamus antibodies in between 35-55% of patients. These antibodies could be producing a condition called autoimmune hypophysitis, or inflammation of the pituitary gland.
- The author propose that a genetic weakness in some ME/CFS patients’ T-helper cells prevents their immune system from being able to quickly recognize and battle the Epstein-Barr virus. As EBV reactivates, the cytotoxic T-cells (CD8) jump in, but lacking the T-helper cells to guide them to the invaders, they shoot wildly, become hyperactivated, and eventually, exhausted. Meanwhile, B-cells start churning out antibodies designed to target the intruder or antigen (pathogen, toxin). Ultimately, they lose their way and attack the pituitary, producing autoimmune hypophysitis (inflammation of the pituitary).
- In a last-ditch effort to bring the pathogens into check, the innate immune system roars into action – producing inflammation and more havoc – but is never able to vanquish the pathogen.
- A world of trouble results. The authors propose that this scenario sets the stage for a wide variety of conditions (reduced Vit. D, insulin resistance, reduced zinc/copper levels, mast cell activation, exercise intolerance, low testosterone, orthostatic intolerance, leaky gut, mitochondrial problems, microglial activation, neurotoxicity, difficulty concentrating, malaise, and the breakdown of the muscles to provide amino acids for energy.)
- Treatment section – In contrast to Avindra Nath, who proposes using checkpoint inhibitors (ICIs) to get the immune system back in gear, these authors think ICIs will make the problem worse. Getting the exhausted T-cells back into action will exacerbate the pituitary inflammation (hypophysitis) and/or the autoimmune disease that caused it. If no infection is present, they believe the treatment can work.
- Depending on what situation a patient is in, the authors recommend different solutions.
- In All Cases – Use supplements to reduce oxidative stress and replenish the compounds depleted by inflammation or malabsorption and to revive the exhausted T-cells. These include vitamin C, n-acetylcysteine (NAC), alpha-lipoic acid (ALA), S-adenosylmethionine (SAM-e), selenium, and B-complex vitamins.
- In Inflammatory and Immunosuppressive States – consider glutamine and nicotinamide mononucleotide (NMN) to restore cytotoxic T-cell functioning, astragalus to help with T-helper cell functioning and EBV reactivation, protein supplementation to maintain protein synthesis, vitamin D supplementation to reduce T-cell hyperactivation and improve insulin sensitivity, and melatonin to protect neurons, reduce oxidative stress and improve sleep. Use antibiotics and antihistamines to reduce inflammation in people with small intestinal bowel overgrowth (SIBO).
- No Pituitary Damage, Normal ACTH levels, and hyporesponsive HPA axis (low cortisol) is present – This may fit some people with ME/CFS. Try supplements that reduce inflammation, and oxidative stress, and stimulate cortisol production, such as ginseng. Add other supplements (see above).
- A Spinal Connection – Although it’s not mentioned in this paper, the spinal-pituitary connection comes from a condition called empty sella syndrome (ESS) – which occurs when the bony structure that holds the pituitary in place becomes flattened. This usually results from high cerebrospinal fluid pressure (intracranial hypertension) – which appears to be common in ME/CFS. In an Unraveled podcast, Dr. Ruhoy reported that she sees ESS or partially empty sella in 80-90% of her craniocervical instability patients.
- Conclusion –
- Both Avindra Nath’s and these researcher hypotheses start from the same place – an infection – propose that an immune defect (in B or T-cells) results in an inability to tackle the infection, immune exhaustion, and autoimmunity/inflammation, and ultimately results in a problem in the brain (TPJ, pituitary gland).
- One proposes an immune approach (checkpoint inhibitors) that the other believes will make things much worse (!). Instead of checkpoint inhibitors, the Spanish researchers focus more on the temporary use of corticosteroids, plus antivirals and a range of supplements to restore T-cell functioning and the HPA axis, reduce oxidative stress, etc.
- With B and T-cells, and even neutrophils and monocytes in ME/CFS all showing signs of immune exhaustion and/or difficulties producing energy, one wonders if problems producing energy is the tie that binds these together.
While Nath ends up in the temporal-parietal junction (TPJ) in the brain, these researchers end up at the pituitary gland in the brain: two immune hypotheses – two brain endpoints…Iteresting!
The Pituitary Gland
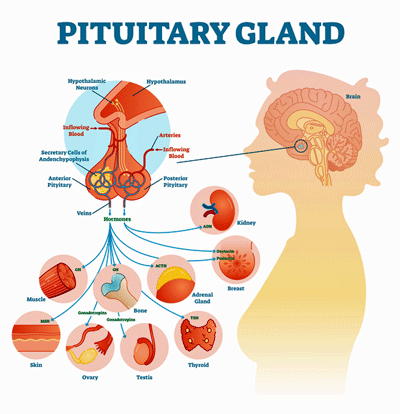
The pituitary gland is part of the HPA axis – one of two major stress response systems in the body. It produces adrenocorticotropic hormone (ACTH) which stimulates the adrenal glands to produce cortisol. In hypopituitarism – the pituitary problem these researchers believe is present in ME/CFS, the pituitary gland doesn’t produce enough ACTH – resulting in low cortisol levels and probably fatigue, inflammation, etc.
Low cortisol levels were one of the first abnormalities found in ME/CFS. Dr. Kaufman stated that virtually everyone with ME/CFS (@90%) has a problem with the hypothalamic-pituitary axis – which could result from intracranial hypertension pounding away at the pituitary. Low cortisol levels have also popped up again several times in long COVID.
It’s never been clear, however, how important a role the various HPA axis abnormalities in ME/CFS play. HPA axis research in ME/CFS flourished early but slowly petered out over time as few firm conclusions were drawn. HPA axis readings continue to pop up but one gets the feeling that researchers were never really able to wrap their hands around what’s going on in the HPA axis in ME/CFS.
Even the low cortisol findings have come under suspicion lately in both ME/CFS and long COVID because of concerns that researchers have not accounted for altered circadian rhythms which may have thrown morning cortisol readings off. (Janet Mullington is exploring this issue in ME/CFS in an Open Medicine Foundation-funded study (blog coming up).
Epstein-Barr Virus
These researchers propose it all begins with a blunted immune response to EBV.
The new paper starts with the recognition that herpes and other viruses can inhibit HPA axis activity, that the pituitary gland’s location makes it particularly vulnerable to infections, and that depending on which way the HPA axis bends, either immunosuppression (high cortisol), or inflammation, or autoimmune diseases (low cortisol) may result.
As with the HPA axis, whether EBV reactivation plays an important role in ME/CFS is still unclear. Increased anti-EBV-dUTPase antibodies, defective B and T cell responses to EBV, increased rates of active EBV infection, and elevated IgM antibodies have all been found, but variable antibody findings, questions regarding active infection, and normal viral EBV loads have made it unclear what effect EBV is having.
The authors propose, though, that EBV provides the crucial connection in ME/CFS. In their 2021 paper, “Epstein-Barr Virus and the Origin of Myalgic Encephalomyelitis or Chronic Fatigue Syndrome“, the same authors proposed that the Th2 immune shift sometimes seen in ME/CFS has left ME/CFS patients vulnerable to EBV reactivation.
It turns out EBV may, in some individuals, target specific cells in the pituitary that regulate ACTH secretion thus reducing it and resulting in low cortisol levels.
ASIA
Indeed, a 2021 Stanford study found increased rates of antipituitary (APA) (33%) and antihypothalamic (AHA) (56%) antibodies that were associated with reduced HPA axis responses. Those antibodies could produce a condition called autoimmune hypophysitis, or inflammation of the pituitary gland. The authors believe that some people with ME/CFS have a condition called adjuvant-induced autoimmune/inflammatory syndrome (ASIA) that’s damaging the pituitary gland.
ASIA popped up in the scientific literature in 2011 when an Israeli immunologist, Yehuda Shoenfeld, proposed that it occurs when genetically susceptible individuals react to one of the “adjuvants” or substances added to vaccines or other compounds to increase their effectiveness. These adjuvants may include things like aluminum salts, emulsions, oils, spike (S) protein, Toll-like receptor agonists, mRNA vaccine lipids, and polyethylene glycol.
Over time, Shoenfield proposed that ASIA is present in diseases like “post-vaccination symptoms”, Gulf war syndrome, sick building syndrome, Sjogren’s Syndrome, and siliconosis – all of which produce some familiar symptoms: low cortisol, chronic fatigue, unrefreshing sleep, sleep disturbances, cognitive problems, and memory loss. The authors of this paper propose that long COVID, ME/CFS and post-COVID-19 vaccine syndrome be added to that list.
One of the most common manifestations of ASIA is autoimmune hypophysitis and adrenocorticotropic hormone (ACTH) deficiency.
Front and Center – Genetic Susceptibility
The authors believe that when a variety of factors (infections, foreign agents, adjuvants in vaccines) interact in a person with a certain genetic makeup (ancestral HLA-II alleles – HLA-DR15), trouble results. Specifically, an inability to ramp up T-helper cells (CD4) not only lets the pathogen replicate but results in compensatory immune responses that produce their own complications.
With the T-helper cells – which alert the immune system to the presence of invaders – unable to recognize the invaders, the latent herpesviruses found in the B-cells come out of hiding and reactivate. The cytotoxic T-cells (CD8) jump in, but lacking the T-helper cells to guide them to the invaders, shoot wildly, become hyperactivated and eventually exhausted. Meanwhile, B-cells start churning out antibodies designed to target the intruder or antigen (pathogen, toxin). Ultimately, they lose their way and attack the pituitary, producing a condition called autoimmune hypophysitis (inflammation of the pituitary).
The authors believe a genetic susceptibility in HLA genes makes it difficult for T-cells to find and fight off EBV.
In a last-ditch effort to bring the pathogens into check, the innate immune system roars into action – producing inflammation and more havoc – but is never able to vanquish the pathogen. (The innate immune system is not designed to rid the body of infections). (A similar scenario of innate immune system activation driven by an underperforming adaptive immune system has been proposed by Avindra Nath – but with dysfunctional B-cells. Since B and T-cells drive the adaptive immune response, one can assume that arm of the immune system i not doing so well in ME/CFS.)
A world of trouble results. The persistent chronic inflammation increases the immune system’s need for vitamin D, and may result in insulin resistance if the vitamin D situation is not addressed. Low zinc and copper levels can also result. Along the way, the authors find a way to hook in mast cell activation, transient hypoglycemia, and exercise intolerance.
The autoimmune hypophysitis produced suppresses ACTH secretion in the pituitary – resulting in low cortisol, and by reducing DHEA and DHEA-s, potentially low testosterone levels as well. The low cortisol produces symptoms like intense fatigue, dizziness, difficulty concentrating, difficulty exerting oneself, and a generalized feeling of malaise.
The pro-inflammatory cytokines unleashed by the innate immune system in combination with the compensatory CD8 T-cell activation may even result in muscle breakdown and the strange pattern found in ME/CF of amino acids being preferentially used to provide energy.
The authors even manage to bring in gut dysfunction, leaky gut syndrome, SIBO, disruption of the blood-brain barrier, reduced tryptophan, oxidative stress, mitochondrial problems, calcium accumulations, anaerobic metabolism, microglial activation, neurotoxicity, and, of course, neuroinflammation.
In shades of Dr. Naviaux’s Dauer hypothesis, the authors believe the immune exhaustion found in post-infectious diseases is not a failure of the immune system but is a compensatory mechanism designed to prevent the spread of intracellular pathogens by denying them the tryptophan they need to replicate.
It’s quite the tour-de-force! By the time we’ve gotten to citation 231, we’re onto the treatment section.
Treatment
Depending on what situation a patient is in, the authors recommend different solutions.
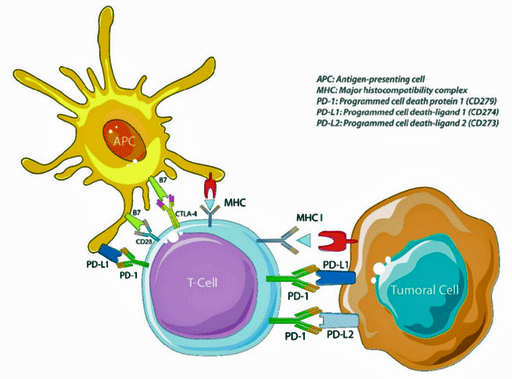
The authors say no to checkpoint inhibitors. (Image by Alexandre-Perrier,-Audrey-Didelot,-Pierre-Laurent-Puig,-Hélène-Blons-and-Simon-Garinet,-CC-BY-4.0. Wikimedia Commons)
No to Checkpoint Inhibitors if infection is present- The authors think that using checkpoint inhibitors – the very drugs that Avindra Nath proposed to be assessed in ME/CFS – to get the exhausted T-cells back into action will exacerbate the pituitary inflammation (hypophysitis) and/or the autoimmune disease that caused it. If no infection is present, they believe the treatment can work.
In All Cases – Use supplements to reduce oxidative stress and replenish the compounds depleted by inflammation or malabsorption and to revive the exhausted T-cells. These include vitamin C, n-acetylcysteine (NAC), alpha-lipoic acid (ALA), S-adenosylmethionine (SAM-e), selenium and B-complex vitamins.
Early Disease Onset Caused by Infection – Use corticosteroids and antivirals at disease onset for a short period of time to reduce the production of antibodies that attack the pituitary, knock down the virus, and reduce the T-cell exhaustion. Knocking down the pathogen is crucial, as they believe it’s the pathogen that is spurring the production of antibodies that are attacking the pituitary.
Consider supplementing with DHEA to assist with ACTH production. Add supplements (see above).
In Inflammatory and Immunosuppressive States – consider glutamine and nicotinamide mononucleotide (NMN) to restore cytotoxic T-cell functioning, astragalus to help with T-helper cell functioning and EBV reactivation, protein supplementation to maintain protein synthesis, vitamin D supplementation to reduce T-cell hyperactivation and improve insulin sensitivity, and melatonin to protect neurons, reduce oxidative stress and improve sleep. Use antibiotics and antihistamines to reduce inflammation in people with small intestinal bowel overgrowth (SIBO).
Disease Onset Not Caused by Infection – use corticosteroids for a short period of time to reduce the production antibodies that attack the pituitary. Consider supplementing with DHEA corticosteroids to inhibit ACTH secretion. Add supplements (see above).
Pituitary Damage has Occurred – If the pituitary has been damaged, corticosteroids may be needed long-term to preserve the functioning of the adrenals. Consider supplementing with DHEA corticosteroids to inhibit ACTH secretion. Add supplements (see above).
No Pituitary Damage, Normal ACTH levels, and hyporesponsive HPA axis (low cortisol) is present – try supplements that reduce inflammation, and oxidative stress, and stimulate cortisol production, such as ginseng. Add other supplements (see above).
Since studies have found low salivary cortisol in ME/CFS but at times normal ACTH levels in some people with ME/CFS, long COVID will fit into this category.
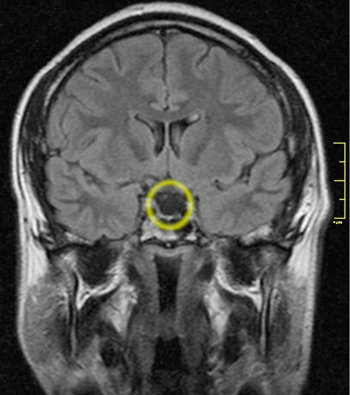
It’s gone! (Image – Empty Sella Syndrome from the Brain by Nevitt_Dilman_CCA_3_Wikimedia_Commons)
The Spinal Connection
Although it’s not mentioned in this paper, the spinal-pituitary connection comes from a condition called empty sella syndrome (ESS) – which occurs when the bony structure that holds the pituitary in place becomes flattened. This usually results from high cerebrospinal fluid pressure (intracranial hypertension) – which appears to be common in ME/CFS.
Hulens believes Intracranial hypertension plays a significant role in some people with ME/CFS and/or fibromyalgia and can be associated with blunted adrenocorticotropic hormone (ACTH), cortisol, growth hormone, luteinizing hormone, and thyroid stimulating hormone responses and an increased prolactin response in these diseases.
In an Unraveled podcast, Dr. Ruhoy reported that she sees ESS or partially empty sella in 80-90% of her craniocervical instability patients. She has also found elevated prolactin in many of her patients which could be linked to the pituitary problems via compression of the hypothalamus stalk as well.
A dysfunctional pituitary gland is the outcome.
Conclusion
Two hypotheses that start from the same place – an infection – propose that an immune defect (in B or T-cells) results in an inability to tackle the infection, immune exhaustion, and autoimmunity/inflammation, and ultimately results in a problem in the brain (TPJ, pituitary gland).
One proposes an immune approach (checkpoint inhibitors) that the other believes will make things much worse (!). Instead of checkpoint inhibitors, the Spanish researchers focus more on the temporary use of corticosteroids, plus antivirals and a range of supplements to restore T-cell functioning and the HPA axis, reduce oxidative stress, etc.
With B and T-cells, and even neutrophils and monocytes, in ME/CFS all showing signs of immune exhaustion and/or difficulties producing energy, one wonders if problems producing energy is the tie that binds these together.






Interesting comment about insulin resistance. A major study of around 1500 ME/CFS patients by the University of Edinburgh – currently in preprint – found three consistent abnormalities:
– chronic low grade inflammation
– insulin resistance
-liver abnormalities (something I have never really heard of in research on the illness – I had mild liver dysfunction in my early years of the illness)
Can you link the pre print please? I have high ATL liver reading and my doctor is going down a rabbit hole. There is evidence of high ATL liver reading in long covid patients. https://pmc.ncbi.nlm.nih.gov/articles/PMC10094195/
https://meassociation.org.uk/2024/08/research-replicated-blood-based-biomarkers-for-me-cfs-not-explained-by-inactivity/
i have had this while a was not drinking alcohol or take medicine.The doctors diddn’*t understand it either. My ACTH was normal and my cortisol was verg high during the day and low in the morning. Strange isn’t it? So. it goes up and down.
Which hospital in Spain is involved in this?
Centro de Investigación Médica Aplicada (CIMA), IdiSNA, Instituto de Investigación Sanitaria de Navarra, Clínica Universidad de Navarra, Pamplona, España
Tom, I had a high ALT reading for decades. I’m sure my ME has been part of it, though I know that my endometriosis was, too (which would not apply to you). But I also want to mention that a substantial part of it is also my Hereditary Hemochromatosis, which I see is being questioned as to whether it can be more present in ME (and is the most common genetic disorder in the US, and is particularly present in men). Although I do think that most people with LC and ME have elevated liver enzymes due to their LC and ME, when investigating, Hemochromatosis has to be ruled out.
I am long term ME patient with one gene for haemochromatosis, type 2 diabetes, and long term high liver enzymes. I completely reversed my liver issues with intermittent fasting, currently trying a keto diet to see if it helps. The proof is in the test results.
I strongly suspect a big issue with us is the Cori cycle. We make too much lactate, its one of the main products of glucose metabolism in us, it goes back to the liver, gets made into sugar, and then raises blood glucose even more. Still not sure I have an answer, but I am testing things.
Just for the record, intermittent fasting does not have to be about weight loss, its about going anabolic/catabolic rather than being anabolic all then time.
Regarding the liver please read here about my story and research : https://www.healthrising.org/blog/2023/10/21/ai-driven-chronic-fatigue-syndrome-clues/
Thanks Efthymios. Sounds like TUDCA helped you? Sounds like it might work on a few things that are ‘out of whack’ in ME/CFS.
https://pmc.ncbi.nlm.nih.gov/articles/PMC4030606/
I have FM. I found that TUDCA and butyrate have helped my gut issues a great deal.
Buenos días, quiero darle las gracias por compartir su magnífico trabajo. Veo en el el futuro de la medicina adaptada la la singularidad y expresiones del paciente. Me gustaría estar al tanto de sus avances en estos programas y resultados.
También darle las felicitaciones por haber encontrado en su caso un tratamiento que le ayudó a paliar los síntomas de sfc. Tal vez para alguno de nosotros sean válidos de igual forma algunos de sus suplementos, empezaré con aquellos que me ayuden a depurar.
En mi caso, la ingesta de DHA y EPA, ha supuesto una mejoría significativa y forman parte de mi rutina diaria.
Me gustaría saber cómo puedo acceder a un estudio personalizado con IA, para saber los factores claves en mi organismo. Le reitero mi agradecimiento.
Google Translate:
Good morning, I want to thank you for sharing your magnificent work. I see in the future of adapted medicine the uniqueness and expressions of the patient. I would like to be aware of your progress in these programs and results.
I also congratulate her for having found in her case a treatment that helped alleviate the symptoms of CFS. Maybe some of our supplements are equally valid for some of us, I will start with those that help me to purify.
In my case, the intake of DHA and EPA has been a significant improvement and they are part of my daily routine.
I would like to know how I can access a personalized study with AI, to know the key factors in my body. I reiterate my gratitude.
I’m hoping Cort will report in that study soon.
I mean “on that study”.
It’s on the list – I think its an important if rather alarming study.
Why alarming, Cort? None of the findings are major, some consistent mild-moderate abnormalities.
I found it comforting. Still no idea what causes the abnormalities. But if we can work on the liver, and on the inflammation, with supplements then we can get some improvements.
Rather than futile waits on lame immune modifying drugs. Ampligen, BC007, Klimas’s long hyped but never completed trials. And the list is much longer. I have reverted to immune system skeptic! Plays a role, for sure, but not a major overriding one
The insulin finding – worried about metabolism and metabolic syndrome – potential precursor to a lot of bad stuff.
My doctor thinks I might have metabolic syndrome. Will be checking labs again in December. What bad stuff do I have to look forward to??
You wouldn’t be alone – a lot of people have it. It increases the risk of some major diseases like heart disease, stroke, and type 2 diabetes. I worry about it in ME/CFS because we get so little exercise. I’m learning about it from Peter Attia. A blog is coming up.
Thanks for your reply. I am actually being evaluated for pre diabetes and have really high, mostly uncontrolled blood pressure. I’ll be on the look out for the blog and will do some research on my own. Having a really rough go this week and don’t see my doctor for another 3 weeks or so.
Don’t forget that eating and dieting is very important to prevent complications. Many people forget this and only think about sports and exercise.
Cort, this made me think of the study of veterans in the US who had had COVID-19 in the US that you reported on a while ago. The researchers found they had higher-than-expected numbers of new cases of Type II diabetes.
I had a brief search on the topic of autoimmunity and Type II diabetes, and it seems there is a possible connection.
But do people with ME/CFS have a higher rate of Type II diabetes, or is it more complicated than that.
I did find a few articles saying that insulin resistance is poorly understood.
I have been puzzling my (small) brain over this study for a couple of weeks.
In one way, it is good. It must be the first decent-sized and well-conducted study showing proper data about a population of people with ME/CFS compared to healthy controls. And – hallelujah – it finds differences that clearly distinguish the two groups.
And yet, why does it show nothing dramatic? Yes, they found abnormalities that are aligned with ill health, but they don’t seem to be severe or highly indicative of something wrong.
My only thought so far is about autonomic problems. Many (most?) autonomic problems do not show up in blood tests. We know there’s an autoimmune or autoinflammatory connection, but so far autoantibodies are teasing us by popping up more than by chance but without being definitive.
I suppose you can go back to “We just aren’t testing for the right biomarker yet”, as experiments with plasma from patients with ME/CFS and nearby conditions did seem to harm healthy cells.
I wonder whether anyone has ever exposed cells to plasma from POTS or OH patients.
Ah, it is too much for me. I look forward to what happens next. And your article, Cort!
Just remembered, there was one study in which rabbits were given a substance that induced production of antibodies suspected of being involved in POTS, and the rabbits subsequently appeared to develop orthostatic tachycardia.
https://pubmed.ncbi.nlm.nih.gov/31547749/
Not quite the same as exposing healthy cells to patient plasma in vivo or in vitro, but the closest I could find.
Yeah I had very similar thoughts.
But I guess that they didn’t test for viruses, or very specific immune traits.
So my ‘hunch’ is the inflammatory and liver abnormalities they found are signals of the immune / viral issues
Ah, good point. As this study seems to be a success, perhaps the same team will be able to do more testing in those areas.
Interesting this mentions liver abnormalities. Additionally interesting the above article mentions low copper/zinc.
I’ve had low copper and zinc bloodwork every year since 2019.
I know they both counter one another so was hesitant to supplement.
Before finally deciding to address the issue with supplements, I felt compelled to ask my doctor to run labs for free copper serum.
Voila!
Turns out my blood copper is low and free serum copper is high.
They were shocked. I wasn’t.
I believe a lot of ME has to do with supporting the liver.
I came so close to supplementing with copper and zinc based on blood levels.
Thank God I didn’t because it’s clearly very high in my free serum and probably would have caused me more damage.
What next is the question…
Where to go from here.
Cleveland Clinic doctors are the worst, IMO.
In conclusion, I think best ways to support our bodies is with liver support and binders.
P.S. Bernie Kosar (former Browns QB) was recently diagnosed with needing a liver transplant and Parkinson’s. He has been a long time ME/mold sufferer and is a Clevelander like myself so I always found a bit of validation of how hardcore this illness is when a tough former NFL player is brought down to his knees by this. Learning of his recent diagnosis, particularly with the liver, was what prompted me to ask my doctor for lab orders to make sure I don’t have liver issues such as Wilson’s Disease before supplementing with copper or zinc. Now most of my blood tests indicate Wilson’s but, as we know how living with ME goes, my urine test didn’t indicate. So the liver specialist said the only way to know for sure is a liver biopsy. Not sure how I feel about that given how amped up our nervous systems can be and how poorly my body heals from wounds.
P.S.S.
Thank you so much, Cort. I love you for all you do to support our cause. God bless you
Hey, Shea, I’ve been evaluated for Wilson’s Disease, too. Though I don’t have it, I know that eye docs can tell if you’re accumulating copper by an eye exam (it creates a ring in the eye). And I do have Hemochromatosis, and they can see if I’m accumulating iron in the liver by doing a liver MRI. Curious whether they can do that with copper too? I go to the Cleveland Clinic.
Thank you! I had the eye exam as a follow up and no copper rings found but they said they wouldn’t really expect to see them.
P.S. Shea, can share my hepatologist’s name with you (at the Cleveland Clinic), if you’re interested, if you’re interested in a second opinion. I had a great one who retired, then a jerk one whom I fired, then found a great one again. The one I have now is sympathetic to ME, and definitely doesn’t want to do anything to make me worse.
Is there a way I can reach you to talk? Thank you!!!
Yes, you can email me at TLEld@aol.com.
Emailing now – thank you!!!!
Timing of your reply couldn’t be more perfect as I have my follow up appointments with hepatology and possible endometriosis appt this coming Monday, so any insight you could offer on any of these topics including the name of your hepatology doc would be amazing.
My liver enzymes are normal.
I take an iron pill every day for anemia that can’t really be pinpointed on labs.
To your point, I really want to avoid any invasive procedures so anything you can offer would be greatly appreciated.
This is very interesting. I have severe ME. Shortly before I got ill, in the seventies, I had glandular fever. That seemed to be when my problems began. I was 18 years old. When I was in my late 20s I was also diagnosed with a hyperprolactinoma caused by a pituitary adenoma. I struggled with unexplained fatigue for decades before I was finally diagnosed with myalgic encephalomyelitis in 2007. I am now 90% bedbound. I would be very happy to be involved in this research if I can be of any assistance.
Which hospital in Spain is involved in this?
Welp, this is so fascinating. Thanks for another great piece.
I recently had a brain MRI and my doc noted that for my age, my pituitary was very large. (No tumor.) I wondered to myself if it was an expression of some sort of inflammation. Who knows, maybe autoimmune hypophysitis. My acute titers for EBV are forever elevated. It’s like I have chronic, acute EBV. My doc says it’s just “background noise.” Again, who knows. Lots to ponder.
“Background noise”? More like immune exhaustion. Get your CD-8 cells checked. That will confirm immune exhaustion.
Thanks!
Great summary of this study, Cort. I don’t know how I’d be able to process the information without your explaining it to me! I find this study a little discouraging (and a bit irrelevent for me, since I’m one of the few people with ME with no EBV antibodies, but I wonder if this could apply to HHV-6, too). I do have low ACTH, and I do believe that the pituitary is a central problem, with me at least. But I, like pretty much everyone I know with ME, have tried NAC and ALA and Glutamine, etc., and they made me worse (I’d love to know whether other people have the same problems with them — they give me better energy and clarity, but cause me extreme pain, and sleep issues). I just feel as though I’ve been here before. P.S. Have had ME since I was 3, with gradual worsening, and then a virus that crashed the whole system 33 years ago today.
“they give me better energy and clarity, but cause me extreme pain, and sleep issues” – that is so interesting. I wonder what is going on there…
I don’t think I put it in the blog but with regard to EBV they also note that EBV dUTPases may be involved which would not show up, I don’t think, in antibody results. These are enzymes that occur during defective attempts at EBV replication which have shown up in ME/CFS. There’s actually quite a bit of work on them in ME/CFS.
https://www.healthrising.org/blog/2017/11/05/crippled-herpesviruses-chronic-fatigue-syndrome/
Thanks, Cort. Interesting!
What about those who did not have a viral infection? Could mold cause the same trouble?
I don’t know. For them it is a combination of a herpesvirus infection and a kind of a hole in the immune system which allows the herpesvirus to attack the pituitary gland – which it appears to have some sort of predilection to do.
If the B-cells, T-cells, the monocytes and neutrophils all have energy production problems – which we don’t know yet as the studies are small – but if they do, one can imagine one would have trouble with all sorts of pathogens.
Yes the immune system targets the ebv but gets confused and goes for glial cells too, fortunately LDN helps with this, as well as opening calcium ion channels.
——- “a vaccine- and chronic infection-induced syndrome behind the origin of long COVID and myalgic encephalomyelitis“ ——- is an interesting title and has me thinking, this could get that odd ball chap Robert F Kennedy interested in funding ME/CFS research. Any whiff of vaccines causing disease and I bet RFK will fund it.
And because ME/CFS is so underfunded already with the appalling decades long status quo of neglect. I’m cautiously anticipate that RFK may boost ME/CFS funding.
He might not be good for the overall health of the US. And I worry about preventable diseases escalating under his watch, especially in children. However I those terrible things aside, I think he could be good for funding ME/CFS and Long Covid and Long Vax research.
Many of us pwME (around 1 in 5) have temporarily worsened or had permanently worsening after vaccination (like myself).
Note: I don’t think it’s the vaccines fault, but our ME/CFS immune system response to them. I’m still pro vaccine.
But vaccines should have been studied on willing volunteers with ME/CFS to figure out why a significant minority of us end up worsening, and a safer way to delver them. Maybe in much smaller doses and stages, than one hard immune hit that knocks some of us for six. Including a less aggressive adjuvant.
Those issues especially vaccine induced ME/CFS like symptoms, may perk up the ears of RFK. And we need to get that message to him.
Question: is anyone aware of RFK’s current position on ME/CFS and Long Covid?
He’s a real wild card! Who knows? If he gets confirmed he might the kind of guy to do this. Time will tell… Let’s hope!
RFK crazy wisdom is precisely what is needed!!!!!
Last month Kennedy accused the FDA on X of suppressing the use of “psychedelics, peptides, stem cells, raw milk, hyperbaric therapies, chelating compounds, ivermectin, hydroxychloroquine, vitamins, clean foods, sunshine, exercise, nutraceuticals and anything else that advances human health and can’t be patented”.
Yes ebv lives in b cells, viruses are cfs root cause, can be managed with low dose naltrexone unconventionally,,every hour, I have followers it works for too, can’t do jack without it. 6mls every hour, takes away fatigue, pain ,pem and pots. You have to be consistent though.
How much is that in milligrams?
Hi Fay, the same, I dilute a 50mg tab in 50mls of water, took me two years of titrating to get this, if yr once a dayer, go to twice, then 3 times etc morn ,lunch,night, then tweak it as needed.
Thanks Vanessa. I’m currently a once-a-dayer. About 5 months in, I did experience a marked improvement in fatigue, which sadly dissipated a few weeks later, but I have continued taking it because it works wonders with pain (unrelated to my ME/CFS) and calms my aged, badly sun-damaged skin, due, I assume, to it’s anti-inflammatory properties. Will try increasing the frequency.
Hi Faye, great, ldn can have many nuances it’s just to learn about it, best to move in the first hour after taking it, if pem or fatigue happens then dose again if it’s been at least a hour or 2 and it will take it away, small amounts of caffiene can support but I can only use coke a cola and choose not too anymore, you don’t really need it , it has the added benefit of helping calcium influx and invoking Cerebral Spinal Fluid flow, but it’s the ldn that’s the real winner, don’t worry if some pain shows at first with increase this won’t take long to pass and not return, I have zero pain anywhere all the time and an life Amazing now!
Ldn tends to slow heart etc, so can be tricky I feel maybe for severe patients and must be monitored, the idea is after you’ve taken it to get moving and build back up, don’t be fooled that it’s cfs fatigue, it can be great for sleep that way, a Calming drug. Let me know if you need more help. All the best. Let us all know how you go, success from another lady I helped here recently,now canning tomatoes lol
Fay,
I am also one of the people who has tried the new protocol for dosing naltrexone. I commented recently here about it. I will re-post it here.
Cort featured the work of the team at Griffiths University in Australia in a post in August of this year, called “Could a Widespread Ion Channelopathy be Causing ME/CFS, Long COVID and Gulf War Illness?”
https://www.healthrising.org/blog/2024/08/20/channelopathy-chronic-fatigue-long-covid-gulf-war-illness/
In this post, Cort highlighted the result that in ME/CFS the TRPMC ion channels are not allowing ions to pass into cells, including into mitochondria. As noted in the study and in his blog post, naltrexone can reverse this block in the ion channels.
The work of the Griffiths group notes that this block often occurs post infection among genetically susceptible individuals.
In the comments, Sue Edwards asked whether or not increased dosing of naltrexone could help. No one responded.
I have since then searched far and wide for evidence that naltrexone can be taken more than once a day. I was surprised at what I found.
I already knew that there were some instances noted in comments on presentations at the LDN Research Trust conferences every year that some people took “low dose naltrexone” in the morning instead of at night, and that some people took LDN in the morning and at night. I also knew that some peope took LDN at doses up to 25mg and found relief.
What I also found out in my search was that there are a lot of people who take naltrexone numerous times a day at different doses. I searched the web in general, Reddit, YouTube, and various patient forums. I found people whose “sweet spot” was 0.0002mg once a week. I found people who took it every other day. Some take it twice a day, and some take it three or even four times a day. One YouTube video consists of a social science researcher in Norway interviewing the two main authors of the work at Griffiths University. He takes naltrexone when he feels that he needs it and some days that is four times a day. He asked the researchers whether or not they recommended this. They refused to answer “because we have not completed clinical trials on this.”
Responses to these dosing regimes range from “I’m going to try that” to “You can’t do that! That’s not low dose naltrexone anymore!” to “When you take it so often, there’s no rebound effect for the release of endorphins, so you’re defeating the whole purpose!” But there are so many people doing so many different things with naltrexone and finding relief, that there just cannot be one way of doing it.
Naltrexone does many things. It’s an anti-inflammatory, an antioxidant, a binder of mu-opioid receptors, an endorphin enhancer, and an opener of ion channels. Maybe people have different conditions that respond to the different actions of naltrexone. Everyone is different, everyone responds differently. People take naltrexone for alcohol addiction at doses of 50mg or 100mg every night, so we know it’s safe even long term.
I do not doubt that people taking natrexone in novel ways are finding efficacy. I cannot be otherwise.
I started taking naltrexone four years ago and veeerrrry slowly ramped my way to 6mg per night where I found that it helped me a lot. So I’ve been there for 3 years now. After looking deeply into the experience of others, I began taking 6mg the mornings, as well.
The first two days were rough, I felt like I’d been hit by a truck or had the flu plus anxiety and depression. The third day was wonderful: the nauseating poisonous ache in my right hip was gone, the swelling in my legs was gone, the anxiety and depression were gone, but I still had some fatigue and PEM.
I upped it to three and then four times a day over the next week. I’m telling you now, this has been life changing for me. Clearly the ion channels in my cells and mitochondria were blocked. When I first take it, I feel warm all over as if blood is getting to places it hasn’t been in a long time. I am not 100% recovered, I’m 76 years old and though I used to be extremely active, I have lost conditioning. I can’t jump back into activity I haven’t done in a long time. I must pace.
Having said all this, I feel better than I have in years. My hairbrush tells me my hair is not falling out at the rate it was. I have no idea if my fingerprints will come back, but at this point I don’t care about that.
This works for me. It will not work for everyone, it can’t possibly do that. I started and stopped naltrexone in the beginning. I went up and I went down in dosage. But I kept trying different ways, different times, different methods (capsules, skin lotion, and finally, dissolved in distilled water). But every time, when I stopped doing it, I got worse again, so I knew it was doing something good. Now I’m glad I kept it up. I’m rather stubborn that way.
I will do anything to keep up my supply of this now. I will tell any prescriber that I’m an alcoholic and I need 100mg tablets of this, one every night, in order for the province (I live in British Columbia) to pay for it – they like to feel they are helping alcoholic addictions anyway.
My hunch is that there is a genetic component to this, plus a precipitating event or series of events. I hope this comment can help other people who are sitting on the fence about trying naltrexone.
That’s what I posted and I’m still using it four times a day. You don’t have to do it every hour like Vanessa does. It’s up to you. If you take 4.5mg every night, then try 2mg the next morning. You won’t feel better at first, you must ramp up very slowly. When you can take 2mg in the mornings in addition to your 4.5mg at night, try 4.5mg in the morning. Then another 4.5mg at noon in addition. Then another 4.5m at supper. Then you’ll see a difference.
After that you can dose at will. You’ll find a regimen that works for you.
Many thanks for re-posting that Ann. I’ll definitely give it a go. I’ve done some experimenting with LDN over the past few years, but never taken it more than once a day. Luckily I’ve had no issues with side effects, and have previously taken up to 12mg per day in one dose.
Taking it more frequently, cost will be an issue for me. I’m in Australia, and have to pay the “private” price for naltrexone as I’m using it off-label. That means $130 per script compared to the pensioner concession price of around $8 I usually pay for scripts. That’s fine currently, as diluting 1 tablet in water, as you also do, a pack of 50mg tablets lasts ages. As far as I can see, nothing over 50mg is available here. With special approval, there is a slow-release implant available here, but then there is no control over the dose per day, so I don’t think that’s a goer. Anyway I’m housebound and effectively bedbound, so couldn’t get to the doctor to get the implant!
I’m a year older than you and have had ME/CFS for 38 years.
I’m aware of the Griffith Uni research, and in fact I was in contact with Professor Sonya Marshall-Gradisnik, the leader of the group, earlier this year (when I heard they were planning a clinical trial, I wanted to be sure they weren’t going to limit it to 3 months!).
Fay, I wish you all the best. Start low and go slow. Yes, cost is an issue for me, too, but it works so well, I will keep up with it. Please come back here and tell us your experience when you try this.
Ann1, is the new protocol still working well for you? Do you know if more will be published about it?
Re the low cortisol and circadian rhythm. That was what struck me immediately when I had my cortisol tested. The people who had ordered the test noticed the high levels at 11am and wanted me to pay them for stress reduction techniques. I looked at my graph and a normal graph and decided that the shape was identical. It’s just that mine was 3 hours later. If it is obvious to an informed layperson, why was it not obvious to researchers? Perhaps it means they did not listen to the patient population when designing the test.
It makes sense that EBV (Glandular fever) could damage the pituitary gland (being a gland). I have low CD-8 suppressor cells and also low testosterone since contracting M.E. / CFS.
Lindy, I’ve had low vitamin D and more recently developed insulin resistance that nearly had me diagnosed with diabetes. (Both my fasting glucose and HbA1c werein diabetic range)
However by pure luck, I tried magnesium threonate for muscle pain and its completely normalised my blood sugars. There’s some studies on this. Might be worth a try.
And thanks Cort for a great article!
Very interesting – HPA axis dysfunction in fibromyalgia too, explaining lots of overlap with ME/CFS. I am wondering though if this hypothesis is correct why there is not a higher prevalence of detectable antibodies against the HPA axis?
Interesting. Formaldehyde exposure also affects T cells. My symptoms all started after coworkers (in hair salon) started using formaldehyde hair straighteners. I’ve used all the supplements mentioned (which do help) and have recently started drinking hydrogen water (selective antioxidant) with even more benefits.
Both the problems with pituitary hpaxis etc and insulin are addressed when using low dose naltrexone unconventionally every hour, the brain and the spleen are rich in calcium ion channels, and this is compromised due to viral infections,,perhaps the bodies silly way to stop more as all viruses enter through these channels, ldn blocks the oipiod receptor and opens calcium ion channels so the body can function correctly again and recieve calicium,spleen deals with insulin etc, brain obviously, everything, off to have another dose now, it has to be used super regular, not once a day! I have followers this works for too.
Ok, ignore my previous comment on immune system skepticism that is more with regards to autoimmune theories.
So here goes on the grand theory / pulling it all together:
1. Begins with an immune system insult – could be viral, bacterial, vaccine, toxin or combination
2. Immune system is activated.
3. Pro inflammatory cytokines result in fatigue etc
4 Allergies common due to over activation
5 over time, immune overactivation turns to t cell exhaustion. Pro inflammatory cytokines hang around, resulting in chronic low grade inflammation. T cell exhaustion leads to reactivation of latent viruses. These cannot be cleared due to t cell exhaustion
6 Initial virus and viral reactivation result in mild-moderate liver dysfunction (which may not contribute to symptomology) and other metabolic changes
7 These things also lead to neuroinflammation and changes to brain chemistry
8 The condition is perpetuated by the t cell exhaustion/ viral reactivation
Perhaps some people get some autoimmune characteristics. But I think this is secondary. Too many people show no signs of autoimmunity, and medications acting on autoimmunity have failed.m (rituximab, bc007)
So, how to treat?
– Treat the t cell exhaustion and/or
– treat the reactivated viruses. PolyBio are trialling two antivirals. I pray for success!
Perhaps some optimal supplement combination can help too
This article begins to shine light on a core treatment dilemma that I have wrestled with for decades; If there is an immune deficiency (perhaps relating to B and T cells), should we try to support and enhance (upregulate) our immune response to finally eradicate the pathogen (virus) that keeps our immune system engaged and exhausted (and causes so many of our symptoms)? OR, should we try to dampen our immune response – to alleviate the symptoms and exhaustion that our already upregulated immune system causes?
This article seems to address this question, by saying “it depends on the patient’s condition – and largely upon whether or not they still have a chronic infection, or just the (pituitary?) damage left by a past infection”. Could this be why some of the treatments suggested can (and do) actually make some people worse? ie. If you dampen your immune response when you are still fighting a pathogen, that could seemingly make you worse. But, if you strengthen the immune response when there is no pathogen to fight, it is only likely to make your symptoms (and exhaustion) worse (and perhaps cause further damage?). Personally, I seem to react negatively to pretty much any supplement that might strengthen my immune response.
I would like to know what clinical tests I could request from my Doc – to know which approach is appropriate for me (bolster or dampen my immune response?), as the article seems to suggest that this could differ from one patient to the next. Treatment trial and error to find out could be deceiving if not dangerous, as herx reactions could mean that initial negative reactions to a treatment may be temporary and necessary to tolerate for long term improvement. On the other hand, initial negative reactions could also mean we are doing the exact opposite of what we should be doing for our personal condition, and would only make us worse. Russian Roulette anyone?
I’d also like to see some kind of trial data for the treatments recommended by these researchers. When they say that a finding “suggests” that a patient should be helped by med or supplement “X”, I appreciate that speculation, but some kind of clinical trials might clarify both the actual potential benefits and potential harms of each of the recommended treatments (and may differentiate which patients that each treatment is appropriate for, and, explain why some patients may get worse).
I’ve always wondered why when I take vit. C it actually causes me to catch a cold.
I’ve trialed vit c. Several times and no matter the brand name ,dose etc. I keep ending up catching a cold/flu about a week after starting🤷♂️
Excellent comment. The immune ‘overactive or under active’ dilemna is one that has consumed me too.
on that, a 2015 (I think) study from Hornig et al found overactivation in ME/CFS patients early in the course of their illness, and underactivation in those many years in to the illness.
Something I have raised before but didn’t get much response to is food allergies. I had lots of food allergies early in the illness ( first 5 years), but over time they disappeared / got much better. There could be several reasons for that, but my preferred reason is a shift in my immunity from overactive to underactive. But here’s another dilemna – I certainly do not catch lots of viruses. Maybe 1-2 colds per year. So is my immune system really under active?
So, I feel that the immune issues might be a bit more nuanced than a binary ‘overactive or underactive’ question.
On treatments, I recall that immune boosting herbs / mushrooms I took early in the illness seemed to exacerbate the allergies. I took them because I thought my condition was viral. Maybe those supplements were not specific enough to eradicate the virus yet still overactivate the immune system?
I have therefore long favoured supplements that help more with inflammation rather than necessarily activating the immune system.
I am quite interested in black seed (nigella sativa). It’s an anti-inflammatory and potentially antiviral. However the research on it doesn’t look high quality.
The immune dilemma is also why I was long wedded to brain / neuroinflammation theories (ie. the issue is the immune system in the CNS rather than periphery). I have gone off those theories a little, though, as the promise in the theory (the Japanese researchers, Jarred Younger) doesn’t seem to have been delivered on. Which makes me think neuroinflammation is definitely a factor, but secondary to a core immune / viral cause.
I hope PolyBio find success with their antiviral trials, but I am not getting hopes up.
I found this whole article very interesting. I was also surprised to read about the pituitary gland and the ASIA syndrome. When I read about it on the link…I was surprised that I filled all the major criteria! I also have Empty Sella Syndrome. I had been taking Dhea but stopped when my GP informed me it can cause cancer..
I have always known how herpes and other viruses inhibit HPA axis activity in ME/CFS from past research, and my TCM doctor has always said likewise. So, what the authors say here seems to be spot on.
And yes, interesting how the brain is always implicated with ME/CFS! And how complex the brain is, and at the same time directly interrelated with the rest of our bodies. Our bodies are like a well-oiled machine, ie. until something goes haywire (like a virus, infection, chemical poisoning, stress, etc.).
Please change the headline!!
When the content of your entire article seeks to establish that it’s Herpes viruses which are driving the disease process, then it’s not helpful to say that it’s the immune response to those pathogens (rather than the pathogens themselves) which is ultimately the problem.
Actually it’s not herpesviruses. They are ubiquitous and the vast majority of people handle them just fine. The authors believe it’s a genetic defect that keeps the T-helper cells from seeing them and leading the immune response. Maybe it would have been better to say “genetic defect” was driving the problem 🙂
I agree with Paul. In the center of this research is the question whether herpes reactivation causes ME/CFS.
It is true that under normal circumstances people handle herpes viruses just fine. But it is also true that it is normal that they break out. When patients undergoing organtransplantation develop severe herpes brain inflammation due to their suppressed immune system the cause for the out-break is also a problem with T-cells. However, the herpes inflammation is real. It’s the problem that the doctors fight with virostatics. The brain damage will be caused by the virus and when they don’t survive it’s the cause of their death. And not a problem with the T-cells.
When we look at the broader picture of ME/CFS research into the question of herpes virus reactivation there is actually more evidence for the simple fact that it is the T-cells themselves that are the locus of herpes reactivation and the reason for why they’re exhausted. This is another reason that I think it doesn’t make sense to speculatively imply a “genetic defect”. What we want to find out is rather what’s the specific and in the beginning often reversible change in the immune system caused by the known ME-triggers like EBV, Covid ect. that allows EBV or the other main suspect HHV-6b to reactivate.
That said, I realise that I don’t think the EBV-reactivation hypothesis in ME/CFS convincing because ME doesn’t look like chronic EBV-reactivation. Also here, I think that HHV-6b smoldering reactivation theory sounds just more convincing to me. And on top, HHV-6b was found in saliva in ME/CFS in amounts according to symptom severity.
Thank you, Cort, for discussing this study.
The disease picture which Prof. Bhupesh Prusty has developed so far, is that in ME/cfs patients, it is the inability to control / suppress the secretion of Herpesvirus dUTPases which is the heart of the problem.
This builds on the long-running research done at the Ohio State University on dUTPases, which began way back in the early 2000’s under Prof Ron Glaser and has been carried on by Profs Marshall Williams and Maria Ariza.
(Check out what Ron Glaser said at the State of the Knowledge workshop on ME/cfs in 2011 – https://videocast.nih.gov/watch=10098 – His talk starts at 1:25:00)
The belief that it is these dUTPase proteins that are driving the disease process, doesn’t imply full-blown reactivation of Herpesviruses, but rather some kind of a genetic susceptibility to these early-stage proteins or an inability to prevent them being secreted.
In his most recent presentation to the 2024 Fatigatio E.V. , Prof. Prusty described what these proteins do to the mitochondria and how they can potentially cause all sorts of problems e.g. vascular endothelial dysfunction.
Of course, what remains to be explained, is precisely how this results in the increase in circulating Fibronectin and the decrease in Natural IgMs which he has reported.
https://www.youtube.com/watch?v=YUnBWvw3wGA&t=1284s
Nicely said! It was so good to see Prusty hook up with those guys. They’ve been on the EBV dUTPase track for a long time.
Hi Paul
Thanks a lot for these explanations and tracking this research back to the naughties. I was aware that that was the case but it wasn’t possible for me to connect the dots.
I read Maria Ariza’s “The Herpes Viruses Are Back” from 2020. However since it is so technical and I am not a natural scientist it was difficult for me to understand the main points of their hypothesis.
But I understood smoldering viral reactivation.
Are you aware that a team around Jackie Cliff from Brunel Uni in London was able to find HHV-6b in saliva proportional to symptom severity in an ME/CFS subgroup and that they are digging into this now?
Originally she’s a specialist for tuberculosis research.
I’ve summarised the disease model which Prof. Prusty has developed so far, in a document that I’ve uploaded to a cloud folder here: https://app.box.com/s/3sgrpzj681unccfhphf81afte1gjzpa7
Found this fascinating & probably very relevant to me. I was surprised that there’s no mention of hypothyroidism in the research. The main (& only test run by the UK NHS) test for establishing thyroid dysfunction is the pituitary hormone, thyroid stimulating hormone. It’s an inexact test for thyroid function really, you need full T4 & T3 tests to establish a complete picture. It’s very poor medicine to solely test the TSH as the NHS does.
I’m hypothyroid & also have many other conditions listed here especially multiple chemical sensitivities & fibro. It sounds really relevant research so I hope it continues .
I use LDN & many supplements including zinc.
As could be expected from the research, I’ve just been diagnosed with full diabetes after years of pre diabetic readings. It’s puzzled me greatly as it’s not my diet. Low carb for 6 months increased my blood sugar reading by 5 points & returning to a slightly higher carb diet raised it another 10.
I shall try increasing vit D & the magnesium theronate etc. Fingers crossed something helps. Metformin has been a nightmare, ruining my already disturbed digestion.
Thank you for the information & explanation, Cort, much appreciated. I will share it with the ThyroidUK forum as I think it has relevance there too.
This article rang many bells for me. Years ago I investigated my hormones to check for any imbalances. For thyroid; Tests showed that I had high levels of reverse T3 (RT3) which is inert but binds to all cell T3 receptors so active thyroid (T3) cannot enter to activate the mitochondria (to give us energy). This can occur in any animal that is sick, (infected or hurt) as part of nature’s way to encourage the animal to rest and heal, by limiting its energy.
For adrenals; My daily cortisol rhythms were reversed with the highest levels at night (= poor sleep), and the lowest levels in the morning (= awakening unrefreshed). Cortisol levels were also lower on average, suggesting adrenal exhaustion.
To check for poor pituitary (or HPA) function in my low cortisol problem, I did an ACTH Exercise Challenge test. I had my baseline ACTH and cortisol levels checked after and while resting. Then I exercised as heavily as I could for about 20 mins (walking up and down stairs). Then I had both measured again as quickly as possible. Both had increased a bit, but not nearly as much as they should have for a normal response. Since ACTH stimulates the adrenals to produce cortisol, my low ACTH response to exercise stress may have explained my low cortisol response. In turn, low cortisol can also be responsible for an inadequate thyroid response (and low energy). I didn’t know how to test the function of the hypothalamus in the HPA axis – which may (in turn) have been responsible for the low pituitary response.
However, I tried several kinds of adrenal and thyroid supplements (together and alone), ramping from low to the highest levels that I could tolerate. These gave me no greater energy (and did not increase my heart rate or low temperature), but high levels made me feel “wired but tired”. So I abandoned my endocrine (hormone) investigations and treatments. When hormone supplements did not help, I surmised that my imbalanced hormones were unlikely to be the root cause of my problems, but rather an effect of a deeper or more basal problem.
Regarding System Exhaustion: The “exhaustion” concept mentioned in this article seems to ring true to me, and in several different systems or ways. ie. The immune response stays upregulated to the point of exhaustion (and is thereby less effective), but it never gears down fully or properly. The chronic exhaustion deregulates and impairs the immune response (B and/or T cells?) and many other systems in the process – while also hijacking and zapping our energy.
Another System Exhaustion: The autonomic nervous system (ANS) which should have a balance between the sympathetic (active/fight or flight) phase and the parasympathetic (rest, rejuvenate, clean and repair) phase – becomes (at least largely) stuck in the sympathetic phase for CFS and Fibro patients – which leaves us exhausted with insufficient rest, rejuvenation, cleansing of metabolic wastes, complete digestion and cell repairs – which can affect virtually all body systems (negatively). The parasympathetic (rest and rejuvenate) phase can kick in (in part) whenever we rest during the day, but mostly occurs during our nightly sleep. This can explain why our sleep is so challenged, and why we awaken exhausted rather than refreshed.
A third system subject to exhaustion: Our HPA axis (and adrenals) may pump out so much cortisol in an attempt to cope with all this chronic stress, that this axis (and our adrenals) can (and seem to) become exhausted, which is accompanied by deregulation and general insufficiency. The low cortisol also down regulates our thyroid function (our gas pedal for energy production in each cell), and deregulates other hormones and many (if not all) other body systems. These are three systems that are known to be (often if not always) exhausted in CFS and Fibro patients.
If this is the case, our systems and bodies might be described as being similar to a long distance runner who is always “in active mode” and can never rest (long enough to rejuvenate back to full or normal function). Thus, they become chronically utterly exhausted and no longer effective in their function (running) at all, with barely enough energy to not completely collapse. This energy deficiency impairs us both physically and mentally.
Could the apparent multi-system(s) exhaustion together cause all of our multi-system symptoms, deregulating a wide array of biochemicals (which also have major regulating effects on each other)?, and, could this leave us in a state of chronic fragility and exhaustion? (Personally, I can’t imagine this NOT being the case).
Notably, the article above suggests that the multi-system deregulation (and exhaustion) might begin with (and be sustained because of) a chronically upregulated (and deregulated) immune response. If this is true, then there would be incredible promise in figuring out how to stop the chronic immune upregulation (and the further deficiencies caused by its exhaustion) – as this may be the key to re-balancing all other systems (which seem quite resistant to individual system balancing treatment initiatives. Most suggested treatments don’t seem to help much for most patients). If an immune system deregulation-imbalance-exhaustion-insufficiency is the core root cause, perhaps it needs to be fixed first before any or all of the other systems can/will re-balance. Personally, I think there is some real hope in this avenue of research investigation.
Thank-you again Cort, for the great synopsis.
Thank you Cort. I was wondering, after reading so much research on your site, especially concerning the brain, why substance p is never mentioned. Unless I missed something on this.
There are numerous papers, mainly, I think from the 80’s on this related mainly to fibromyalgia. Substance p was then associated with dysfunction of neutrophils, glial cells, HPA axis, pituitary, histamine, gut inflammation, anxiety..whew! And more.
Please check it out. You can google- substance P in fibromyalgia, or similar heading-gut inflammation and substance p, etc. It’s associated with Raynard’s syndrome, which I have along with the fibromyalgia. Sorry to not have included links, but is easy to find with a quick search.
What do you think though? This not mentioned in a lot of brain focused research. Too old a research to focus on this, or forgotten, overlooked? I’m perplexed. Wouldn’t it be included as an issue here also? Thanks!
Hello. I live in Suffolk UK. Where on earth am I going to find a practitioner who’s up to speed with all this? Robin.
I don’t know but our Doctor Rewview program will be released soon.
Any chance of a story on PolyBio’s antiviral studies, Cort?
https://www.bloomberg.com/news/newsletters/2024-07-29/treatments-for-long-covid-draw-on-decades-long-hiv-research
Thanks for this article Cort. It’s an interesting study, with an elegant theory. The variation in biological states intuitively makes sense, and that could help explain the large variation in symptomology. And also varied response to treatment.
I think my daughter is in the ‘Inflammatory and Immunosuppressive’ state. She seems to catch cold after cold.
Something I have been thinking she might try, which isn’t mentioned in the research, is nigella sativa (black cumin). Research shows that it reduces inflammation and boosts immunity. It has also been shown to help sleep. In theory, all these things are what we want in supplements for ME/CFS, and Anecdotally I have heard some good things about it’s use in ME/CFS.
This is so fascinating… I was just reading an article about Mast Cell Activation Syndrome (MCAS). They describe MCAS as an exhaustion of both the B-cells and T-cells of the immune system, causing the “innate immune system” to ramp up to fight off pathogens (which is not its job), increasing release of histamines (which cause allergies and hives) and cytokines (which create excessive inflammation in the body). That is my non-scientist understanding and I am open to being corrected :).
It’s interesting because that is exactly what is described in this article– could MCAS and ME/CFS be pretty much the same thing?
Ha! Mast cell activation as well! That fits right in there… Good to hear….
Hi Chris. Interesting “dovetail” Can you provide a link to the article you mentioned?
mastattack.org
Thank you!
Gracias Cort Johnson . Ayer conocí esta página cuando compartieron este artículo en redes.
No he podido parar de leer 🙂 Tengo SFC desde hace 29 años, contraje con 28 años mononucleosis me dió bastante fuerte, desde entonces hubo un antes y un después.
Y he empeorado, tras un periodo de fuerte estrés prolongado 3 años, y también han influido los cambios hormonales de la edad (soy mujer).
Así que aquí me encuentro, buscando qué hacer. Como ya sabes incluso los supuestos especialistas en la materia, me refiero a médicos, tampoco tienen mucha idea y no escuchan.
Aunque he estado leyendo , no me he podido enterar de apenas nada… entre otras cosas ahora mismo tengo covid por añadidura. No tengo formación en ninguna de estas áreas, pero tengo que hallar respuestas para mi, porque quiero vivir y no sobrevivir.
De acuerdo a lo que he leído en el foro , cada quien tiene mejorías con tratamientos diferentes ajustados a su persona. Lo que es válido para unos puede no serlo para otros. En este sentido, la propuesta de la ayuda de la IA para que elabore nuestro perfil y de ahí nuestro tratamiento me parece el futuro en el diagnóstico y tratamiento de forma simultánea. No me cabe duda que en se irán implementando .
Para los que no somos tan afortunados de contar con esa ayuda de IA, ¿qué podemos hacer? de momento, hago como todos con ensayo error con suplementos.
Y quería hacerle otra pregunta, ¿ me puede decir algo de los siguientes suplementos? he estado buscando en el foro sin éxito.
NA+ Triptófano
BPC157 (péptidos) y KPV (peptidos). Por más que busco información de cómo actúan en nuestro organismo, no lo encuentro. Gracias!!
Hi Cort: As always, many thanks for the blog! I read it a few times and then read the Study. Checked my DNA info from 23andMe, and as it turns out, I do have the gene and the troublesome variant (A). This so perfectly fits my experience, save one exception that I’ll mention toward the end of my response. My first ME/CFS/whatever it was experience was late perimenopause. I went to very high functioning to immense suffering and dragging myself through the days in a few short months. There may have been more than one pathogen implicated, but to make a 5-year story very short, root cause diagnosis was Lyme/bart/babesia (and yes, I did have the definitive EM rash for Lyme). Treatment, albeit with a couple of short relapses that remitted with short treatment courses, was heavy duty oral abx pulsed. I did get well. And stayed well until my 2nd COVID booster and then probable COVID itself. I did react to the first 3 shots, but not strongly. Still struggling to get well and this blog/Study explains a lot.
I do want to mention something about Vitamin D. About two years into my first crash, a well-meaning doc prescribed 50,000i.u. of ergocalciferol. It took me a while to figure it out, but it WORSENED symptoms substantially. Thyroid antibodies went through the roof, serum calcium frankly elevated, PTH suppressed, and active form D substantially elevated. I stopped D, but am still so sensitive to external supplementation that even milk with concentrations over my usual brand, I’ll react to (even if I’m unaware of the switch). I tracked that when I relapsed, active form D was elevated with storage form low. Upon treatment, that normalized. So, it’s my pet theory that “low” D actually isn’t, low storage is present due to overconversion of D from its storage form to active. I’ve tracked this many times, and while I’m a anecdotal of “1”, we know this happens in T.B., Lyme, and other infections. Chris Kresser did a podcast on this phenomenon a while back and there are other similar observations out there.
Just throwing out food for thought. The “osteoporosis” mentioned in the blog and Study may very well be connected to the overconversion. When a doc suggests D and testing, I insist on measuring both storage and active form. I won’t supplement with high active form, which is almost always the case (or high normal) when I’m feeling badly.
Wow – isn’t that something – well until the 2nd booster shot (and maybe COVID) and heavy duty abx worked! Thanks for the fascinating look at Vit D…Everything in its right proportions…