

Geoff’s Narrations
Treatment Takeaways
The Blog
Treatment Takeaways
- “Treatment Takeaways” will now be a feature on the blogs.
- Jen found her way to health via a cytokine panel, a doctor willing to get creative, and a lot of persistence. Cytokine panels can theoretically be ordered by patients. I was having trouble, however, finding one in Quest or Labcorp that was available. Maybe others will have better luck.
- Approved in 2019, Rinvoq is one of nine JAK inhibitors approved for use in the U.S – three of which were approved in the last two years. These drugs work inside the cell to turn off the signals that spark the cell to produce inflammation. They can inhibit the production of many immune factors including most of the interleukins and all the interferons.
- These wide-ranging drugs have been shown to improve the joints in rheumatoid arthritis and psoriatic arthritis, the gut in Crohn’s disease and ulcerative colitis, the spine in ankylosing spondylitis, and the skin in atopic dermatitis.
- Even though Jen’s illness began with an infection, and testing showed that she’d been exposed to multiple pathogens, pathogens weren’t her problem – the immune hyperactivation that the pathogens triggered was. Jen appears to fit into the “hit and run” hypothesis of ME/CFS where a pathogen triggers a long-lasting immune dysfunction.
- Jen’s system simply needed to be reset and once she found the right drug it reset itself amazingly quickly – and then – and here’s the really good part – it has remained reset. Even though she’d been largely bedbound for almost two decades no long-term damage appeared to have been done.
- Jen probably fits a specific subset of ME/CFS patients – those with increased cytokine levels. We know that everyone doesn’t fit this profile because ME/CFS cytokine studies have had such variable results. Plus her results also seem at odds with findings of T and B-cell exhaustion, and studies suggesting that pathogen persistence may be triggering long COVID.
- That’s not surprising- we should expect multiple immune subsets in these diseases.Jen’s story is an example of what precision medicine; that is medicine driven by testing results and not purely by disease status – can achieve.
- Long COVID has triggered interest in powerful immune drugs that never saw the light of day in ME/CFS – and clinical trials are underway. The best hope for rapid movement lies with the rejiggered RECOVER initiative which, with its recent $500 million outlay, has promised to take a more creative approach to long COVID clinical trials. Time will tell – but if the initiative follows through it could fairly quickly provide data on a wide array of drugs.
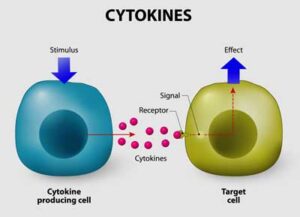
Jen’s cytokine panel indicated she needed to bring them down.
The Crucial Test
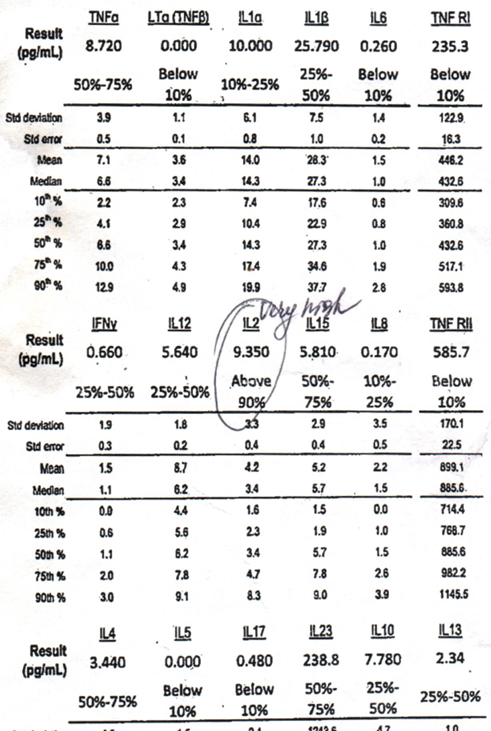
The results from a cytokine multiplex 18-panel test gave Jen the target she needed to find the drug that helped her. These panels typically assess a range of cytokines involved in immune responses and inflammation.
Some of the cytokines commonly included in such a panel are:
- Tumor Necrosis Factor Alpha (TNF-α)
- Interleukin-1 Beta (IL-1β)
- Interleukin-2 (IL-2)
- Interleukin-4 (IL-4)
- Interleukin-6 (IL-6)
- Interleukin-10 (IL-10)
- Interleukin-12 (IL-12)
- Interleukin-13 (IL-13)
- Interleukin-17 (IL-17)
- Interferon Gamma (IFN-γ)
- Interleukin-18 (IL-18)
- Monocyte Chemoattractant Protein-1 (MCP-1)
- Macrophage Inflammatory Protein-1α (MIP-1α)
- Granulocyte Macrophage Colony-Stimulating Factor (GM-CSF)
- Interferon Alpha (IFN-α)
- Interferon Beta (IFN-β)
- CCL3/MIP-1α
- CCL2/MCP-1
Note that a doctor’s prescription is not needed to order this test. (To get an insurance company to pay for it, yes, but not to order it). Finding one may not be so easy, though. Quest Diagnostics says they provide a cytokine panel (but not in my area). An AI search stated that Labcorp does (but I could not find it).
The Drug
Upadacitinib, or Rinvoq, the drug that proved so helpful, is a selective Janus kinase (JAK) inhibitor approved by the US Food and Drug Administration, the European Medicines Agency, and other agencies around the world to treat chronic inflammatory and/or autoimmune diseases such as rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, atopic dermatitis, ulcerative colitis, Crohn’s disease.
JAK inhibitors work inside the cell to stop it from producing inflammatory molecules.
Approved in 2019, Rinvoq is a relatively new drug. It’s one of nine JAK inhibitors approved for use in the U.S. JAK-inhibiting drugs appear to be a growth field: three were approved in the last two years and other, potentially more potent, drugs are being developed.
These drugs work inside the cell to turn off the signals that spark the cell to produce inflammation. They do this by shutting down enzymes called Janus kinases which trigger the JAK-STAT pathway. This pathway gets activated when an immune factor – a cytokine – lands on a receptor on the outside of a cell. That receptor turns on the JAK-STAT pathway which activates inflammatory genes in the nucleus of the cell, causing it to produce inflammatory molecules.
This pathway is involved in a wide range of inflammatory activities. Rinvoq is able to inhibit the production of many immune factors, including most of the interleukins (IL-2, IL-4, IL-6, IL-7, IL-9, IL-10, IL-12, IL-13, IL-15, IL-21, and IL-23), all the interferons (IFN-α, IFN-β, and IFN-γ), and growth factors (erythropoietin (EPO), thrombopoietin (TPO), and granulocyte colony-stimulating factor (G-CSF)).
Rinvoq is particularly effective at blocking the JAK1 enzyme, but since the 4 JAK enzymes work cooperatively, blocking one may help block others. It demonstrates as well as any drug how one drug can be helpful in so many different diseases. By tamping down inflammation it’s been shown improve the joints in rheumatoid arthritis and psoriatic arthritis, the gut in Crohn’s disease and ulcerative colitis, the spine in ankylosing spondylitis, and the skin in atopic dermatitis.
JAK inhibitors are being trialed in a wide range of disorders including ulcerative colitis, systemic lupus erythematosus, giant cell arteritis, eosinophilic disorders, diabetes, leukemia, alopecia areata and allergic disorders, uveitis, inflammatory rheumatism, etc.
Exactly where in Jen’s body Rinvoq halted the inflammation is an intriguing and important question.

Putting a stop to her cytokine storm worked for Jen.
Immune Suppression Works
One of the first things to know about these drugs is that they are immune suppressants. Despite the fact that Jen’s illness began with an infection, and testing showed that she’d been exposed to multiple pathogens, the pathogens weren’t the problem. If they had been, she likely would have gotten worse. (One of the most common side effects is an increase in upper respiratory infections.)
Instead, she appears to fit into the “hit and run” hypothesis of ME/CFS where a pathogen triggers a long-lasting immune dysfunction. The pathogen probably quickly became irrelevant and, as her attempts with antivirals showed, a treatment dead end. Instead, her testing showed that a hyperactive immune response was her problem.

Jen needed to reset her system, and once she did, it went back to operating normally.
Rapid System Reset – Even after 18 Years
Jen’s system simply needed to be reset, and once she found the right drug, it reset itself amazingly quickly – and then – and here’s the really good part – it has remained reset.
Her case seems reminiscent of Dr. Klimas’s model of ME/CFS as a disease in which a pathogen or toxin or whatever knocks ME/CFS patients’ systems out of their healthy, stable setpoint into a new, unhealthy but still stable setpoint. It takes work to get out of that setpoint – hence the need for the drug – but if you can move it back into its old healthy stable setpoint, you’re back to normal functioning.
Jen’s story suggests that despite her long and very arduous illness, no lasting harm was done – a hopeful conclusion.
The JAK Inhibitor ME/CFS / Long-COVID Connection
Jen’s wasn’t the first time that JAK inhibitors have shown up in long COVID or ME/CFS.
Wes Ely’s big baricitinib (Oluminant) REVERSE LC trial will thoroughly examine a different but similar JAK1 inhibitor. Olumiant and Rinvoq are both used to treat rheumatoid arthritis and skin conditions. The side effects – upper respiratory tract infections, nausea, and increased liver enzymes – are similar as well. While Rinvoq inhibits both JAK1 and JAK 3, Olumiant, which AI reported “had a slightly different efficacy profile”, primarily inhibits JAK1 and JAK2.
The 5-year 500-person NIH-funded study is underway at six universities (Emory University, University of California, San Francisco, University of Minnesota, Vanderbilt University, Yale University, and University of Pennsylvania) in the U.S.
Ely’s interest in baricitinib (Olumiant) dates back to a June 2020 study that used artificial intelligence to tag the JAK1/JAK2 inhibitor as a possible COVID-19 drug. The AI study, interestingly, predicted that Olumiant would suppress both inflammation and the coronavirus. (This is because Olumiant suppresses the kinases the coronavirus uses to propagate.)
Robert Phair has proposed that JAK-STAT inhibitors could block the IFN-a upregulation that may be happening. “WoodyRob” on the Phoenix Rising forums reported that Janet Dafoe recently tweeted that an ME/CFS patient improved on another JAK inhibitor, filgotinib.
A recent study that illuminated the “muscle-brain” connection, and specifically mentioned long COVID, proposed that STAT inhibitors such as ruxolitinib be trialed in long COVID. They believe these drugs could prevent the “muscle dysfunction associated with chronic and infectious diseases.”
Specific Subset
Please note that Jen probably fits a specific subset of ME/CFS patients – those with increased cytokine levels.
We know that everyone doesn’t fit this profile because the ME/CFS cytokine studies have had such variable results. Plus, her results also seem at odds with findings of T and B-cell exhaustion which – according to an AI query – should produce reduced cytokine levels. (The checkpoint inhibitors Avindra Nath proposed to unleash the immune system might have had disastrous results in ME/CFS.) Long-COVID studies and the monoclonal antibody results suggest that the virus, or parts of the virus, may be causing problems as well. In short, a number of different immune dysfunctions could be present.
ME/CFS and long COVID, of course, are heterogeneous diseases and we should expect different subsets to have differing immune profiles. Note how different a panel I had done in 2017 was. I have more low than high levels and only one really high cytokine (IL-2). My immune profile is something like high IL-2 levels, reactivated HHV-6, high IgG 4, and very low NK cell cytotoxicity. My partner’s panel is different from mine.
The Future
All of which calls for precision medicine; that is, medicine driven by testing results and not purely by disease status – which is what Jen received. The heterogeneity found in post-infectious diseases suggests that clinical trials should be accompanied by extensive biological testing to determine which patients most benefit from them and why. The Open Medicine Foundation’s LIFT study is an example of a study that’s doing that.
Thankfully, powerful immune drugs are being considered and tested in long COVID that were never allowed to see the light of day in ME/CFS or fibromyalgia. It makes sense that a powerful drug might be needed to address a powerful disease. Tony Komaroff’s 1996 study, showed, after all, that ME/CFS was significantly more functionally impairing than multiple sclerosis, heart disease, and others. People with these diseases should be able to take a shot at more powerful drugs.
That said, the NIH will still not let researchers apply for ME/CFS clinical trials via its special ME/CFS grant mechanism. (The research center’s grant will allow clinical trials.)
Research on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) (R01 Clinical Trials Not Allowed)
Long COVID, though, has sparked more interest in these drugs. Monoclonal antibodies that produced similarly rapid and complete remissions in long COVID indicated that, as with Jen, the right drug in the right patient can produce wonders. At least three small long-COVID monoclonal antibody trials (one by Nancy Klimas) are underway.
IVIG is another drug that has been used with mixed effects in ME/CFS but can work very well in the right patient. An IVIG study is underway in the RECOVER Initiative, but the study unfortunately is not digging into the participants’ biologics. (The Paxlovid trial does, to some extent).
Besides these studies, two stem cell studies, Xiflam (a novel small molecule inhibitor and anti-inflammatory), the ensitrelvir antiviral (Henrich), HIV drugs (tenofovir disoproxil/emtricitabine and Selzentry (Putrino), anakinra and immunoadsorption are being trialed.

Long COVID is opening up treatment possibilities for post-infectious diseases.
These are nice starts, but we need much more. The NIH and the RECOVER Initiative (gulp) are where we once again have to look for rapid progress. The story of the RECOVER Initiative is not done, but thus far, its ponderous, top-down, uncreative approach appears to have displayed in brilliant colors all the shortcomings of the NIH.
After a horrific start that ate up hundreds of millions of dollars, the clinical trials section of the Initiative appears to have made a turnaround, however. Pledging to engage in small, research-intensive trials, and to use platform trials, RECOVER could fairly quickly provide patients the data they need to get their doctors to try many new treatment options. Time will tell, but if the RECOVER Initiative can switch gears, the future looks bright.
As that’s happening, Jen’s case study, when published, will provide a powerful reminder that when the right drug meets the right patient, miracles can happen and should spur doctors to do the appropriate testing and try the appropriate drugs.
Jen’s case is also a reminder of the important role that genetic studies can and should play in these illnesses. Family studies can provide powerful insights into these diseases, yet despite a heritability study which found evidence that ME/CFS runs in families, I’ve never seen an ME/CFS family study. That’s the kind of study that occurs as a matter of course in other diseases. On that note, it was good to hear that the ME/CFS Roadmap Initiative spurred the formation of a genetics group at the NIH, that Ian Lipkin’s group is doing its own GWAS study, and of course, that the DeCodeME study is underway.

Publishing Jen’s story was just the start. Next came digging into it and trying to figure out what it meant.
Donation Drive Update
Thanks to the over 100 people who have supported Health Rising thus far!
Presenting Jen’s remarkable story was only the beginning. Next came digging into it and trying to understand what it meant for ME/CFS, long COVID, etc. Why? Because that’s what we do – we put on our thinking caps and dig, dig, dig. If you find that more comprehensive approach to these illnesses helpful, please support us in a manner that works for you.






Super interesting Cort and congrats to Jen! Just curious if you’re going to try Rinvoq? , since you have high IL2 . I have the same as you high IL2 – CD5 elevation with high HHV6 and EBV. Do you know if her doctor was a Rheumatologist or ME/CFS specialist ? Also wondering if this would improve sleep ?
Also if someone can explain , if the JAK inhibitors don’t cross the Blood Brain Barrier due to large molecules , how did it knock down her neuroinflammation? Was always told that pathogens or toxins crossed over to nervous system through leaky BBBand embedded, so that’s what flipped the switch . So if rinvoq or any of the JAK & TNF blockers can’t effectively pass through , how does it work on the CNS immune system to resolve ? Or is this just the body symptoms ?
I believe that Rinvoq is lipid soluble so able to cross the blood brain barrier.
I don’t know who her doctor was – whoever she/he was they were willing to explore. I asked two AI programs how to lower IL-2 levels. One mentioned cyclosporine and the other corticosteroids, methotrexate, or biologics (e.g., TNF inhibitors, IL inhibitors) may reduce immune overactivity, indirectly lowering IL-2.
Theoretically Rinvoq could help as it can lower many cytokine levels. If I had a shot at it I don’t know. Since I have more low than high levels I would probably see if I could find a specific IL-2 inhibitor. First, I would do a series of cytokine panels, though, to see if the high levels held. My high IgG 4 levels have – so I know that’s a real thing.
You do good work Cort. However, it would be good if you perhaps could probe some of the researchers a bit harder. You mention Klimas – again. She’s been talking about immune dysfunction and trials with immunomodulators for YEARS, with very little to show for it. Why? Can you probe a bit harder?
Was the basis for her theories flimsy? Is funding an issue? Were her theories good but haven’t been supported by her research over the past few years?
I am always willing to keep an open mind, but what’s going on? I would be delighted if my skepticism on Klimas has been misplaced, and she delivers meaningfully on her promise!
The likes of PolyBio seem to be able to move with some urgency…
And on the positive front, Simmaron said their initial results on their rapamycin trial are ‘very promising’
https://www.bbc.com/future/article/20241119-long-fatigue-the-exhaustion-that-lingers-after-an-infection?fbclid=IwY2xjawGt2D5leHRuA2FlbQIxMQABHbrhc5yPJnWZj57RK0qlbog1S937C9CwIfqBnuRHk-_gxAaMKXaZ-L_NgQ_aem_e7P4_hZw6DQXiCaBkeWrNQ
Thank you for Jen’s recovery story. I wish I could get excited but even if we found a drug that might help us, finding a doctor to prescribe it is another story. I had been willing to try an HIV drug that was prescribed for me by a certain researcher years ago but gave up after contacting 5 different doctors who said no. I live in the Midwest where everything is approach from a conservative viewpoint. I’m probably leaving out important points in this study, but I feel like I just can’t comprehend anything anymore. I’m running out of hope and out of time( age wise). I think I’m going on year 10 of being severely fatigued and sick. I give monthly because how would we ever know what is going on without your podcast. Forgive me if I don’t make complete sense. I’m just tired, like really tired.
Finding doctors who will experiment can be so difficult. Our Doctor Review program will be up shortly and hopefully that will help.
Publishing Jen’s case study will be something we can point to – and then there’s the Wes Ely’s big study and other immunomodulatory studies. Ely’s study is so big that it could immediately be a game-changer for long COVID if it works out.
Let’s see what happens with RECOVER’s new clinical trials as well. While they are long COVID and not ME/CFS if successful we should benefit.
In short, the arc is pointing upwards maybe not as quickly as we like but definitely upwards. Don’t give up hope!
Thanks for your support!
Good to hear about Rapamycin. I believe the oxaloacetate results were pretty good as well – a paper is coming out.
I don’t know what’s happened with Dr. Klimas’s etanercept, Mifepristone trial. I;ve been calling it the trial that always seems about to begin! My understanding that it was fully funded. I had an interview with her a couple of months ago which will be posted shortly. I’m sure I must have asked her about it.
Provisional on oxaloacetate :
https://www.frontiersin.org/journals/neurology/articles/10.3389/fneur.2024.1483876/abstract
From searching on Google, could only see a couple of commercially available oxaloacetate supplements. Very expensive, and not sure about the credibility of the companies supplying it. Think will wait a bit on this one before considering trying!!!
I just spent an hour in annoying AI ‘voice jail’ trying to get a live agent to talk to at Quest Diagnostics. After trying several numbers I found a number that can at least lead to voice mail with the right navigation, which was to say I was a physician and request ‘supplies’. It’s 1-866-697-8378. I’m hoping to somehow access the cytokine panel from Canada, perhaps through the mail. Or make a trip down there one day if I’m well enough (ironic!).
Geoff I am also in Canada. Please share if you find any leads!
Helen
helenfcg@gmail.com
I recently self-ordered the at-home “CytoDx Cytokine Analysis” assay from a company called Diagnostic Solutions (https://www.holisticheal.com/cytodx-cdx-test-kit.html). From everything I can tell, the company is legitimate and well-regarded. A mobile nurse came to my home to do the blood-draw. It was easy-peasy, albeit somewhat expensive.
That sounds great! But you’re not in Canada though, eh?
I have taken a similar drug Xeljanz and it helped me a lot. I did have to stop it after a year or so because of side effects. I’ve also improved on other immunosuppressant drugs (methotrexate) but again side effects became an issue. I was on Imuran for several years but then it stopped working. I have been on prednisone (10-15 mg a day) for about 10 years. Without it I am basically bed and couch ridden. With it I still require about 4 hours of bedrest each day and am very limited even when out of bed.
My rheumatologist believes my ME is from a hyperactive immune system. My ME was triggered quite suddenly by radiation therapy for cancer. It is a dirty little secret that radiation treatment can cause ME. 🤦🏽♀️
Hi Eileen, the fact you took radiation is very highly indicative you could have the LI-FRAUMENI SYNDROME which comes with WASF3 Wilkott-. Aldrich.
I have one mutation heterozygous for the TP53 LI-FRAUMENI GENE.
I did the 23andme health ancestry home saliva test it came up in the drug reactions list on the Genetic Genie downloaded for free or a donation.
ekelly101@optonline.net
Thx! I did see a genetic counselor who tested me for the ATM gene and there was some sort of possible abnormality but nothing definitive. That was in 2010 and the test was only done in CA.
I will look into this further.
Interestingly, I did have radiation therapy last year for squamous skin cancer stage 3. I did not have any significant problems; just typical redness, swelling and fatigue for a few weeks. My Radiation oncologist said that the skin cancer site treated was small and the radiation does not go deep, whereas breast cancer radiation covers a much larger surface and goes very deep so much more damage.
ok I hope you get some answers soon, all the best wishes to you Eileen xx
I went on AI search regarding Jak Inhibitors and ME/CFS. Guess what Cort, It directed me to Healthrising!!!
😄
Good AI! Love it! 🙂
I am in the US, and I was able to have my cytokine levels checked via a mail-in kit from Radiance Diagnostics. You order the kit yourself, pay a small fee to have the blood drawn at a participating location, and then immediately mail the collection tubes back (with an ice pack) via FedEx expedited shipping – must be done early in the week so the sample arrives in time to process. The cytokine panel is $460, and they also offer an immune subset panel for PASC for $540 which looks at lymphocytes, etc. This is of course not covered by insurance, but could give you some valuable insights that you might be able to bring to an open minded provider for consideration. https://theradiancediagnostics.com/catalog/
Thank you, Cort.
Fact is that we know next to nothing about cytokines in ME/CFS. These molecules have short half-lifes and are being produced in response to immune challenges. It is therefore to be expected that their titers may be completely different between, let´s say, clinical baseline and PEM. These fluctuations (which can be enormous) could be one of the reasons why measuring cytokines in ME/CFS patient groups has not yielded any meaningful results.
It is sad to say but it´s true: we still have no systematic longitudinal description of ME/CFS – and this haunts ME/CFS research again and again.
Yes, so much variability with cytokines. I took heart, though, that Jen was able to use her cytokines to find a good drug option and track hers successfully over time and many biologics specifically target cytokines. It’s a bit of a quandry! I think you might need a series of consistent results to have a chance at these drugs.
Can Jen supply her cytokinin values and the lab where she submitted her samples so that we can compare apples to apples? It would be great if she could do this before her case report comes out since that may take a while. Thank you.
This is a very interesting remark:
Just checked AI and it’s really short:
“Many cytokines have a short half-life, typically ranging from minutes to a few hours.”
Heightened cytokine levels during PEM seems very plausible, just going off my gut feeling. During a PEM crash, I literally feel a lot of inflammation (e.g. joints, muscles, brain, etc) and pain.
And it might also be (partially) the reason of the flu-ish type symptoms ??
Part 2 To add to my previous comment PEM vs Stabile:
– there is research
https://www.nature.com/articles/s41598-018-20941-w
“- Our study has three main findings. First, we have found that exercise can be associated with significant changes in cytokine profile that are still observed 18 hours following symptom-limited exercise. Second, our study suggests that exercise may allow better discrimination of ME/CFS case status than resting values. Third, we have found that cardiac structure at baseline and cardiorespiratory responses following exercise with a one-day protocol do not appear to distinguish cases of ME/CFS from healthy sedentary controls.”
https://www.s4me.info/threads/value-of-circulating-cytokine-profiling-during-submaximal-exercise-testing-in-myalgic-encephalomyelitis-chronic-fatigue-syndrome-2018-montoya-et-al.2345/
Thanks for pointing this out. Exercise studies are great windows into what may happen at the beginning of PEM. And we also need them!!!
What I was referring to and what I am particularly missing is what you may call the “100 heroes study”: following 100 (just to throw in a number) ME/CFS patients (a sizable portion severe) longitudinally for let´s say a few months, digital monitoring, accelerometry, step counts, HRV etc. included. Whenever there is a significant change in baseline these patients get a specified work-up of urine, blood, saliva with a contracting local MD or a visiting nurse. Thus we whould obtain a zillion of longitudinal data points which are tagged with individual clinical descriptors.
This would be the first serious longitudinal study in ME/CFS ever (currently we only have some case reports).
That’s my dream study! I would throw in an exercise test at some point. This should be the next big NIH study. They “owe” us for doing half their promised intramural study and for whacking the research centers so badly. We need sustained support from the NIH and we’re not even getting that at the current low levels. C’mon guys!
Jarred Younger has been working on the data from a big NIH-funded ‘good day, bad day” study which included lots of immune factors. Hopefully we’ll hear about that soon enough.
I second that !
What would it cost?
– 10–20 millions ?
Do does this mean there is no value in having cytokines checked? I don’t understand how Jen could track her levels and do anything meaningful if the levels would be different on a blood draw just a few hours later.Do we know what levels there are, and variability, in a healthy population?
I can not tell you that, I am not an immunologist. But reading the literature cytokines are very volatile and I am sure that during periods of viral reactivation you will have a different profile than during calm periods. On the other hand, there are normative data (ranges) for *healthy* people for interleukins. However, ME/CFS patients are not healthy people. This is why I continue to suggest longitudinal studies in ME/CFS, which have been sorely missing so far.
Ideally the range given by the testing laboratory would take into account the variations in the healthy population.
Lots of values differ widely in healthy people and we still manage to measure them, although whether this has been achieved for cytokines, I don’t know.
If it hasn’t been achieved yet, that may be why cytokine testing of this kind is generally looked upon as unreliable at this stage.
Jen’s recovery with Rinvoq sounds very interesting, but the dosage she took is not mentioned. Do you have any idea what dosage of Rinvoq she was taking?
She said that she took 15mg of Rinvoq per day.
Cort, thank you so much for posting this.
What you wrote fits beautifully with my situation (and, I hope, with a substantial portion of other ME/CFS patients). The pathogen that triggered my illness (in my case, a severe candida infection 40 years ago) has long been dormant, but its impact on the immune system lingers on.
I recently self-ordered a cytokine assay, and it showed that I have exceedingly high levels of GM-CSF, IFN-gamma, IL-2, IL-6, IL-15, IL-18, and IL-5.
It’s extremely encouraging that a drug like Rinvoq may permanently reset the immune system to its normal state.
Hi Cort,
The “treatment takeaway” is playing the 17 minute “blog” narrative for me. Sorry, if others have already pointed this out, but I’m having text block comprehension challenges today.
Thanks!
Cort- Thank you for asking Dr. Klimas about her ongoing study…I would like to know the results on the GWS study but also what is happening on the ME/CFS study which was resently funded. The Pandemic halted the earlier funding. Also Dr. Klimas, a few years ago, found the difference between Gulf War Syndrome and ME/CFS was that the FWS people had immune systems on over drive but ME/CFS patients had close to not functioning immune systems … she did a deep dive into this kind of testing I believe. I would be curious what she thinks about all this. Before finding those results, she could not tell the difference by symptomology of the two conditions and she had many patients with these conditions.
I wish I could get immunological testing. I don’t know whether it would lead anywhere, but it might. Right now I am taking conventional DMARDs and hoping they will help.
As an Aussie, it would be very hard to get my blood sent overseas for testing.
There is an immunologist in Melbourne who apparently takes a close look at this type of thing, although whether it would lead to treatment I don’t know.
(I have auto-immune OCHOS, as described by Peter Novak, which reduces blood flow to the brain via abnormal vasoconstriction. People with ME/CFS may have this as part of their illness. Dr Novak mentions diagnosing a few patients with ME/CFS with OCHOS in his paper.)
JAK inhibitors come with serious side effect risks:
https://www.drugs.com/comments/upadacitinib/
Clinical trials would clarify the risks and allow people to make informed decisions if JAK inhibitors prove effective for ME/CFS and similar conditions.
Many years ago when I had refractive ulcerative colitis, JAK inhibitors and biologics weren’t available, so I didn’t have to make this decision. Ended up having to have my colon removed after high doses of steroids didn’t work.
I wonder wheher I would have tried them. Sadly I did not get the best advice about UC even for the treatments available back then.
Thie research certainly supports this & the interrelationship of the kinases makes targeting & unintended downstream consequences a challenge.
OTOH, there seem to be plenty of doctors prepared to rx for children with immmune related alopecia. I wonder if they genuinely have a different view of the risk profile as I have not seen a reliably targeted JAK in the literature.
Hi everyone,
After a long time away from participating but following, I saw that this blog by Jen X and Cort about her recovery story provided some valuable information to complete a potential pathway I suspected for a long time: ME/CFS, in many cases that are related to EBV or even sizeable EBV reactivation, appears to be at a crossroad that can lead to things like either Multiple Sclerose, other auto-immune diseases, EBV related types of cancers and ME/CFS.
In other words: ME/CFS could be in many cases “the Dauer” (as introduced by dr. Naviaux) inhibiting against the *real modern day risk of several auto-immune diseases
and several types of cancer*.
The common clue would be EBNA specific T-cells creating a very strong immune response to the EBNA proteins that EBV infected cells produce during latency. It has much potential to produce such strong immune loop that it is devastating.
Immune exhaustion, increased interferon and itaconate and paradoxilly enough frequent modest EBV reactivation could dampen that dangerous immune loop. Basically ME/CFS would provide many components to avert excessive escallation of this hypothesised immune problem.
The case is made on the forum, with links to relevant scientific research below. The link below. It is written fairly condensed and not very easy to read. Putting the idea out is important for now.
Kind regards to all. Back to my “participation inhibition” for a time to come.
https://www.healthrising.org/forums/threads/ebna-trained-immunity-crossroad-hypothesis.6913/
I’m wondering if Jen tried Enbrel too?
Last year I was able to trial it for a month, pretty sure I felt worse but now I’m wondering if maybe I gave up on it too early.
I have a high TNF-a and 1 other one IL but don’t remember.
Family studies can provide powerful insights into these diseases, yet despite a heritability study which found evidence that ME/CFS runs in families, I’ve never seen an ME/CFS family study.
Hi Cort, Family studies have always interested me. I live in Australia. In approx 1990 Andrew Lloyd and Dr Ian Hickie from Sydney, RPH, did research Genetic testing on 4-5 family members of mine looking for a genetic marker for CFS.
Results – The frequency of HLA haplotype sharing among affected siblings was significantly increased above expected (p<0.001). The prevalence of HLA DR4 was increased in the family index cases (p<0.01). Affected-pedigree-member and lod score analyses also supported linkage.
I am sure by now much more has been done on the genetics of ME/CFS.
Professor Sonya Marshall-Gradisnik is Director of the National Centre for Neuroimmunology and Emerging Diseases (NCNED), Griffith University has done a lot of work in this area.
It would be great to revisit the family genetics again
I’m also in Australia and I wish there were family studies being done now! Myself and all 3 children have ME/Dysautonomia etc. One daughter was mostly bedbound for 10 years from age 11, my son has been bedbound for 5 years since age 15. I am 56 and have been moderate/severe for years and mostly homebound/bedbound, and 1 daughter is mild/moderate!!! I’m a single parent and their sole carer – we desperately need treatment options!! We went from being a super fit, active family scampering around living on an island in Fiji with mild Dysautonomia symptoms to one by one being completely incapacitated following viral infections (many years before Covid).
Sorry to hear that Vivienne. I am wondering if you changed environments at all since your family got sick. Like different home or city. I wonder if an environmental factor was the trigger.
Also did you look into hyperchromatosis? It is genetic and runs in families. The symptoms are like CFS/ME. Highly treatable. But it appears that PWCs can have mid-high values like me (500) which maybe just due to chronic inflammation. So one needs to discern if such levels should be treated or not. The treatment is not expensive.
So basically this article is saying that ME/CFS is increased cytokines. If that were the case, then pwME who have had cancer treatment that knocks out the cytokines would have recovered by now. Sorry but I can’t see that abnormally high cytokines are the answer.
It is saying that one person’s ME/CFS was caused by an increase in a certain cytokine, not all cases.
so many anecdotal stories and small studies about recovering ME patients. There must be some truth in it somewhere. However, we must be very careful with this because natural progression and many other factors play a role. It is clear to me that ME is a very heterogeneous disease. The difficult thing is to determine the cause for each patient. In any case, it is always nice when patients improve. And what is very important is the duration of the disease. If you have not been ill for very long, you have a better chance of recovery. Rest is a very important factor. Only we as patients don’t get that.What has become clear to me over the past 30 years is that autonomic nervous system and immune system are the driving force behind this disease. That completes the circle.
Dr Bruce Patterson has been looking at high cytokines in ME and long Covid. I took his cytokine panel when I was feeling well in remission of ME/CFS and it came back with some abnormally high cytokines. I asked why this would be if I were feeling well and in remission but I never got an answer. So I don’t believe that cytokines are the reason why this person got better. She could have just got better for no reason.
I also did Dr. Bruce Patterson cytokine Long Covid panel in 2021, when it was just appearing. I had the following high markers:
TNF-ALPHA – 20.0 HIGH pg/mL < 11.0)
SCD40L – 20533.6 HIGH pg/mL < 9236.0
CCL5 (RANTES) – 13982.0 HIGH pg/mL < 11800.0
IL-10 – 5.8 HIGH pg/mL < 1.0
IFN-GAMMA – 3.7 HIGH pg/mL < 3.5
I haven't followed their recommended protocol for treatment with a statin, HIV antivirals and something else. My immunologist was not keen to dampen some of the markers without knowing the underlying reason for the immune system to be this active. Instead, he tested and found a thrombophilia – Protein S Deficiency. I took supplements and anticoagulants / NSAIDs to address this PROS1 deficiency.
A year later, in 2022, I had Quest's Cytokine 13 Panel done (ordered by a Dr. who was located in California) https://ltd.aruplab.com/Tests/Pub/0051394 – this test came back with all normal values. In a twist, I had this test a month after my only PCR- and antibodies documented Covid infection. I was very symptomatic. Yet, my antibodies faded by the 3rd week of illness like it had never happened.
Prior to all that Cytokine testing, in 2015ish I realized I was dealing with cytokines and mast cells. My baby was having issues, too, and was diagnosed with mastocytoma with normal tryptase. Long story short, I took OTC actions to reduce cytokines (for example, stop taking eldeberry) and found some relief taking Resveratrol a natural JAK inhibitor.
I feel getting cytokines under better control was helpful for the course of my ME/CFS but I can't say I am fixed. I am very curious to know more about the lab that Jen used and other markers and tests and supplements.
As others here have pointed out, Rinvoq is a nasty drug that could put you in the hospital or even 6 feet under if you’re not careful.
But Rinvoq has a cousin who is gentler and nicer (i.e., safer), called Sotyktu (deucravacitinib). Sotyktu is indicated for the treatment of psoriasis. With its ability to tamp down an overactive immune system, I wonder if Sotyktu could potentially help some ME/CFS patients?
I’ve given up all hope, I no longer have the energy to try new things, I’m just going to drink myself to death over the holidays.
Why go on?…
Jason, I am so sorry that you are in despair. Perhaps I comprehend your hopelessness at least a little because I have found myself feeling similarly at various points over the years. It’s hard not to give up at such times.
Reaching out for help can feel pointless, and honestly, not even a desire because that would not fit our goal/decision/intention of ending our suffering right now. I know it’s hard to imagine right now but a step in the direction you desire will have its own life long consequences. Please reconsider. Please. Please. Please. Do reach out for help right now. One option is to call 988 Suicide and Crisis Lifeline
And the question is, WHY go on?
Here’s some reasoning that’s kept me going and maybe will find it helpful as well. It briefly considers three points of reason for your consideration and something else as well, a hope and a means to cope.
If reading is beyond you right now, it is available as an audio download _ scroll down to bottom of webpage where you can download it as an audio file.
Why Go On?
https://www.jw.org/en/library/magazines/g201404/
I hope this helps you reconsider Jason. You can plug any topic/question into the search box on this website to find something of help/interest to you.
Please do take care. It’s dark now. There is light at the end of the tunnel – light not yet in your view – but it is there. Truly it is.
I recently read on an ME forum someone say something to the effect of: why would one give up now? The future looks promising, there’s so much going on. There will be a treatment or cure some day and that day will be the best day of your life! It’ll be euphoric! Don’t you want to live the best day of your life?
I know it’s ridiculously hard with severe ME to get through each day. I too feel like giving up sometimes but lately I’ve been thinking, OK, it’s one day of hell at a time until the best day of my life.
I spoke to an ME practitioner who could retire now but she said it’s so exciting that there’s no way she will 🙂
Hi Cort, this seems quite encouraging. My adult son has had severe ME/CFS for 5 years and would like to be tested for cytokine presence. His primary care doctor seems reticent to acknowledge the disease and search for treatment options that fit my son’s ME/CFS modalities. Does Healthrising have a ME Practisioner POC that we could provide to our doctor for guidance in diagnosis, specifically the possibility of testing for the cytokines and treatment with JAK STAT inhibitors? Any help would be appreciated!
Does anyone know of a Cytokine panel test in the UK?
Where can you get this type of blood work/I
am in Canada
hi, do you know the reasons of your remission from me? What is that helped you, after how many years? Thank you
Hodgkins Lymphoma blood Cancer RINVOQ has been used, treatment is 8 weeks
They now suspect I have Hodgkins Lymphoma my ALT 48 UL is raised so is Alkaline Phosphatase 132 UL & my D-LACTATE is high 480 UL a normal range is 120-246 UL.
I’m now waiting on an Ultrasound of my Liver
Aidan, could you please rewrite your post to make your point clearer? It’s a bit confusing as it stands—almost feels like a cliffhanger.
Carlos Chalbaud, all I meant was RINOVIQ is used in Hodgkins
Lymphoma Cancer as a treatment in my comment above
I checked a lot of my genome results, I do not show any JAK types or Wilkott-Aldrich genes but I do have the TP53 Gene mutation c.215C>G for L-Fraumeni.
I know TP53 is also one of 6 genes in MDS Myelodysplasia Syndrome.
I have a mutation for Acute Myeloid Leukemia maturation.
Does anyone on here know what treatment was used for the woman in the NIH for the TP53 &Wilkott-Aldrich’s findings by Dr. Hwang? thank you