

Geoff’s Narrations
The GIST
The Blog
A trip to Seattle to see Dr. Ruhoy held up the blogs a bit but they’re back!

Wust assessed the effects of exercise on heart rate variability.
Exercise physiologist Rob Wust’s “Wearable heart rate variability monitoring identifies autonomic dysfunction and thresholds for post-exertional malaise in Long COVID” study did something simple but important: it tracked heart rate variability (HRV) while assessing activity levels, and before and after exercise, and during daily activities to get a sense of how the autonomic nervous system in long-COVID patients stood up to exertion.
In general, we want high HRV readings. Note, though, that HRV levels are highly individual: what is considered high or low for one individual might be different for another. The key is observing how HRV levels fluctuate over time and in relation to exertion.
Low HRV levels suggest that sympathetic nervous system dominance is present, aka a “fight or flight” process. That, of course, comports well with the “wired but tired” symptoms found in these diseases.
The HRV test tracks a known pattern: our heart rate speeds up when we inhale and slows down when we exhale. The inhalation triggers SNS activation while the exhalation triggers the parasympathetic nervous system.
Since we both inhale and exhale more when we exercise, an exercise test provides a nice glimpse into how well our autonomic nervous system handles stress. A healthy, flexible cardiovascular system should be characterized by a heart that’s able to speed up and slow down rapidly during the long inhalations/exhalations during exercise.
The GIST
- Exercise physiologist Rob Wust’s Wearable heart rate variability monitoring identifies autonomic dysfunction and thresholds for post-exertional malaise in Long COVID” study did something simple but important: it tracked heart rate variability (HRV) while assessing activity levels and before and after exercise and during daily activities to get a sense of how the autonomic nervous system in long COVID patients stood up to exertion.
- In general, we want high HRV readings. Low HRV levels suggest that sympathetic nervous system dominance, aka a “fight or flight” process, is present and that the parasympathetic nervous system arm of the autonomic nervous system has failed to kick in.
- This nice-sized (127—Long COVID; 21 healthy controls) study tracked HRV during numerous activities, every hour for 24 hours after exercise, and during sleep.
- The authors also used the exercise study to recommend heart rates for each patient that would keep them below their anaerobic threshold—the point at which the body increasingly relies upon the vastly less efficient and ultimately toxic mode of anaerobic energy production.
- Heart rate variability (HRV) levels were lower in patients with long COVID compared to healthy controls across all activities and during sleep. While HRV levels recovered after strenuous exercise in the healthy controls within 3-6 hours, they remained lower for a remarkable 24 hours in long COVID patients, indicating that a state of postexertional malaise had been reached. Mild exercise – exercise done at a heart rate 80-90% of VTI (anaerobic threshold) – was better tolerated with HRV levels recovering (to a still lower level) within 5-6 hours.
- Over 40 percent of long-term COVID patients recorded heart rates exceeding their anaerobic threshold during their daily activities; i.e., they were exerting too much.
- Intense exercise didn’t affect nighttime HRV levels in the healthy controls, but dramatically reduced them in the long COVID patients.
- ME/CFs and fibromyalgia studies have also found that intense exertion impacts the autonomic nervous system negatively. For instance, the parasympathetic nervous system should quickly brake the sympathetic nervous system and return heart rates to normal. While in the healthy controls, they did, ten minutes after exercise, the heart rates of the ME/CFS patients remained significantly elevated.
- A similar process appears to be present regarding mental exertion.
- Long COVID patients who engaged in more than 60 minutes of exercise, regardless of intensity, experienced greater reductions in HRV than patients who engaged in 20 minutes of exercise.
- Even short periods of intense exercise (>100% of their safe heart rate), though, were associated with “abnormal diurnal adjustments of HRV in patients”. i.e., abnormally reduced low HRV at night.
- Studies suggest increased sympathetic nervous system activity may be behind ME/CFS patients’ poor sleep. An inability of the parasympathetic nervous system to kick in and cool down the sympathetic nervous system during the deepest sleep stages may keep people with these diseases from experiencing the “recuperation of energy” that sleep normally brings.
The Study
This nice-sized (127-long COVID; 21-healthy controls) study went well beyond others in the length of time it tracked HRV (several days) and the detail with which it tracked it. It assessed HRV during numerous activities (sleep, eating, travel, relaxation, work, walking, exercise, household chores, etc.), every hour for 24 hours, after intense exercise, and nighttime after exercise.
The authors also used the exercise study to recommend heart rates for each patient that would keep them below their anaerobic threshold—the point at which the body increasingly relies upon the vastly less efficient and, ultimately, toxic mode of anaerobic energy production.
Results
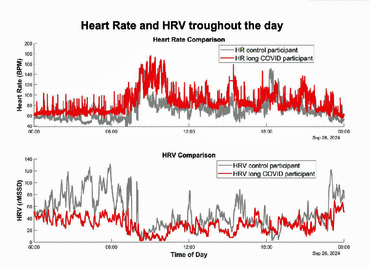
Notice the higher heart rates during the day (red – top graph) and lower heart rate variability (red – bottom graph).
As expected, patients with long COVID had lower power output and oxygen uptake consumption at VT1 than controls.
Heart rate variability (HRV) levels were lower in patients with long COVID than in healthy controls across all activities and during sleep. While HRV levels recovered after strenuous exercise in the healthy controls within 3-6 hours, they remained lower for a remarkable 24 hours in long-COVID patients, indicating that a state of post-exertional malaise had been reached. Mild exercise (done at a heart rate 80-90% of VTI (anaerobic threshold) was better tolerated with HRV levels recovering (to a still lower level) within 5-6 hours.
Over 40 percent of long-term COVID patients recorded heart rates exceeding their anaerobic threshold during their daily activities, indicating that they were exerting too much.
Interestingly, HRV dropped the most in mildly affected patients but not as much in “moderately affected” patients, presumably because the HRV levels in the moderately affected patients had already bottomed out and simply could not go lower. It took 10-13 hours for the HRV levels in moderately affected patients to increase after exercise intensities above 80% (80% is considered mild).
Exercise and the Autonomic Nervous System in Chronic Fatigue Syndrome (ME/CFS) and Fibromyalgia (FM)
Several studies demonstrate that too much exertion produces an autonomic nervous system hit in ME/CFS and FM.
A 40-person 2022 study, “Reduced Parasympathetic Reactivation during Recovery from Exercise in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome,” found normal HRV ratios during rest and exercise but reduced values overall, suggesting that both the sympathetic and parasympathetic nervous systems had taken a hit. Perhaps in a sign of chronotropic incompetence, ME/CFS patients failed to attain normal heart rates during exercise.
It was what happened after the exercise, however, that produced the most interest. Once exercise stops, the parasympathetic nervous system should quickly brake the sympathetic nervous system and return heart rates to normal. While in the healthy controls, they did, ten minutes after exercise, the heart rates of the ME/CFS patients remained significantly elevated. The authors concluded that the results “demonstrate(d) a reduced functional capacity for exercise.”
Likewise, a recent fibromyalgia study also found the same strange pattern of decreased heart rates during exercise and increased heart rates 30, 120, 180, 300, and 600 seconds after exercise.
While it may not seem inconsequential that heart rates don’t quickly return to normal, this finding appears to uncover a fundamental weakness. A 1999 study that followed almost 2,500 people found that a delayed decrease in the heart rate during the first minute after exercise was “a powerful predictor of overall mortality”.
The relative risk of dying in the participants (average age 57) doubled over the next six years in those with delayed heart rate recoveries. This did NOT mean they were likely to die, but their risk of dying had increased. Since then, several studies have shown that quick heart rate recovery after exercise is a function of a healthy and responsive parasympathetic nervous system.
A similar process appears to be playing out with mental exertion. Japanese researchers have shown that sympathetic nervous system activity spikes during a cognitive test. Once the test is over, though, the parasympathetic nervous system should step in to shut the sympathetic nervous system down, and return the brain and body to a rested state. The failure of the parasympathetic nervous system to activate after the cognitive test was done was highly correlated with fatigue.
Nighttime Heart Rate Variability
Intense exercise didn’t affect nighttime HRV levels in the healthy controls but dramatically reduced them in the long-term COVID patients. The decrease in the long-term COVID patients’ HRV during sleep may help explain why it’s so difficult to get a good night’s sleep after too much physical exertion.
Tracking activity levels over time enabled the researchers to determine how different activities affected HRV levels at night. People who engaged in “intense exercise” ( >100% of their “safe heart rate”) for less than 60 minutes experienced significantly reduced HRV levels compared to people who engaged in mild exercise (80-90% of “safe heart rate”). In a further warning not to engage in longer periods of exertion, long-COVID patients who engaged in more than 60 minutes of exercise, regardless of intensity, experienced greater reductions in HRV than patients who engaged in 20 minutes of exercise.
Even short periods of intense exercise (>100% of their safe heart rate), though, were associated with “abnormal diurnal adjustments of HRV in patients”; i.e., abnormally reduced low HRV at night.
The takeaway: short periods of intense exertion and long periods of exertion at varying intensity hit HRV levels at night particularly hard.
The Sleep HRV (Autonomic Nervous System) Connection

Intense or long-duration exercise at milder intensities particularly affected HRV levels at night.
The sleep problems in ME/CFS have been a mystery for a while. Despite the problems with unrefreshing sleep, a 2011 sleep study reported that ME/CFS studies have not revealed any substantive evidence indicative of a primary sleep disorder…”. Ditto, a 2011 review concluded “there is no demonstrable neurophysiological correlate to substantiate a basic deficit in sleep function in CFS.”, Then a 2012 review concluded that “polysomnographic and other objective measures of sleep” have observed few differences in sleep parameters. Once again, ME/CFS seemed to evade the researchers’ grasp.
Natelson, however, pointed out that non-EEG studies might be more effective in assessing sleep quality as far back as 1998. By 1997, researchers had shown that ‘autonomic activation’ could induce sleepiness during the daytime. Still, it wasn’t until 2007 that researchers explored how the autonomic nervous system might impact sleep in ME/CFS.
Boneva started it off with a 2007 CDC study, which found higher heart rates, reduced HRV, and high norepinephrine levels during sleep, suggesting that a “state of sympathetic ANS predominance prevailed”. Next, a 2011 Australian study, which found no differences in ‘classical’ sleep tests, found evidence of reduced nighttime heart rate variability. That same year, Wyller’s 2011 pediatric study found evidence of sympathetic nervous system (fight or flight) activation (increased blood pressure, heart rate) precisely when the body should relax and recover during sleep.
The study oted that “the low HRV strongly predicted sleep quality, suggesting a pervasive state of nocturnal sympathetic hypervigilance in CFS Vollmer-Conna,
Natelson’s 2013 study found no significant changes in sleep architecture (time spent in various stages of sleep), but significant changes in all heart rate variability measures between the two groups during sleep. When they looked deeper, they found that patients who felt sleepier had a ‘higher fractal scaling index’, suggesting that ‘micro-arousals’ during non-REM or deep sleep were being undetected in traditional sleep studies.
Looking deeper, a small 2018 Finnish study found that the expected gradual increase in parasympathetic activity as the participants entered slow-wave sleep was missing in people with ME/CFS. They concluded that “parasympathetic functioning during deep restorative sleep seems to be impaired in… CFS.”
The parasympathetic nervous system’s inability to “de-arouse” the autonomic nervous system during the deepest sleep stage may prevent energy recuperation.
An even deeper dive into parasympathetic nervous system activity by Australians in a 2020 at-home sleep study found no reductions in sleep time or sleep stages. However, it revealed reduced parasympathetic nervous system activity during the deeper stages of sleep (NREM2 and slow wave sleep), which was correlated with self-reported sleep quality and well-being.
The authors proposed that a missing “autonomic de-arousal” during slow-wave sleep in the ME/CFS patients prevented them from experiencing the “recuperation of energy” that normally occurs during slow-wave sleep, in particular. Perhaps in an attempt to compensate, people with ME/CFS actually experienced more slow-wave sleep than the healthy controls in the study, but the crucial element—the quality of their slow-wave sleep—was lacking.
During slow-wave sleep – the deepest stage of non-REM sleep – brain glucose consumption (energy consumption) drops significantly, the brain experiences greatly reduced neuronal activity, and goes into repair mode.
Using HRV to Improve Your Health
Heart Rate Variability (HRV) An Underused ME/CFS/FM Management Tool: PT II – Surveying the Landscape
Conclusion
All signs point to the fact that too much exertion – particularly intense and longer duration physical (as well as mental exertion) – disrupts autonomic nervous system (ANS) functioning – in people with long COVID and ME/CFS/FM by putting them into a fight or flight (enhanced sympathetic nervous system) state. Milder exercise is better tolerated but it can take the ANS 24 hours to recover after intense exercise.
ANS dysfunction, then takes its place alongside the remarkable series of dysfunctions (reduced gene expression, metabolite and protein production, extracellular vesicles, reduced energy production) associated with exercise.
Interestingly, a similar type of parasympathetic withdrawal that fails to “de-arouse” the sympathetic nervous system during the deepest states of sleep may hold the key to the unrefreshing sleep that people with these diseases experience. Problems with the stress response, then, may impact many areas on these diseases.
- Coming up – Improving parasympathetic nervous system activity during slow-wave sleep.






Does anyone know how to dial the ANS down? I’ve tried multiple different meditation techniques, nothing seems to fix the issue.
I do find the non exercise technique of Yoga Nidra mindfulness very helpful for slowing heart rate. But makes no difference to energy levels. Very calming though.
But when I fall asleep at night I often wake up sicker in the morning with internal tremors. It feels like a vibration throughout my torso and legs. Like my nerves are firing or muscles are trembling for no reason. But I’m pretty sure there’s no actual muscle movement. It’s more a nerve sensation. But like Cort mentioned it’s during sleep I think the ANS isn’t settling down. So I need a medication to help that
Clonazepam has helped for that, but is addictive so I don’t want to rely on that, so only take it for the worst days.
But surely there are other medications people know of?
Because the various mind therapies people have recommended haven’t worked for me. And the don’t work while I’m asleep.
Noting no anxiety and no racing mind. They aren’t even an issue for me. I have the version of ME/CFS that is a very physical disease. i.e. Exertion worsens it. That’s it. Emotional stress doesn’t.
“I often wake up sicker in the morning with internal tremors. It feels like a vibration throughout my torso and legs. Like my nerves are firing or muscles are trembling for no reason.” – it sure sounds like something is getting activated….
I’m just looking into it but researchers are working on some things to activate the parasympathetic nervous system during slow waves sleep.
I guess you’ve probably tried Flexeril? The muscle agitation made me think of it?
i personally took flexeril at least 35 yrs ago when not much was known & i was pushed to exercise & then could barely stand and took flexeril at nite-over time that med was really depressing & I wouldn’t want that experience for my worst enemy
B Rob when I get that kind of orthostatic tremor its a sign that I’ve been overdoing it. I focus what energy I have on active rest, rather than ADLs. Playful creative activities, gentle music and time in nature all work for me. Check out the 7 types of rest. Maybe reduce your caffeine intake if you drink caffeine? I have fewer tremor if I have one coffee in the morning. I eventually worked out its not even the 2nd coffee that does the damage, its the over-exertion that makes me want the 2nd coffee. YMMV
Hi Rob, I am glad you find yoga nidra helpful. I am a (Buddhist) meditation practitioner since 15 years and the teachings and the exercises from the Zen and Vipassana schools have proved to be the most important pillar of support for me since I fell ill with ME/CFS moderately in 2020.
When I read your comment the first thing that came up for me was the question whether you might have a peripheral inflammation of the nerves as it is common in a subgroup of ME patients (and the reason why ME has the word “myalgic” in it which means muscle (nerves?) pain.
What you might read as a psychophysical disfunction of the ANS might actually be a very material incapacity of your nerves to function properly because they are under fire. If that were the case I’d recommend that you search elsewhere for an effective solution.
Except for subjective reports of heroic monks who claim to have defeated serious chronic illnesses with meditation we don’t know any meditation practices with which you could fight an acute inflammation process in the body. Even the Buddha had to rest when he caught a cold.
As fas as my experience goes and what the literature tells us consistently: in ME/CFS the most effective tool is a systematically practiced health management with pace and rest at the core.
I have ME/CFS of the type that involves brain inflammation. I got the relapses under control with pacing and have been pretty stable for the last two years.
During and after the brain inflammations my sleep was highly incapacitated. But because I knew that it must be difficult for my damaged brain to get itself into sleeping mode I had the patience to just lie in bed patiently for hours until it would find its way to sleep. As soon as my brain started to heal after I had brought the flare-ups under complete control my problems with sleep got better as well slowly by themselves.
The patience and the insight in these processes were a direct result of my regular mindfulness meditation practice for many years prior to falling ill. I can recommend that you stick with an ordinary mindfullness practice like “breath as the anchor” for fifteen minutes daily. It might not bring you the quick results which you underststandably hope for but you will benefit greatly in the long run.
The reason is that the kind of transformation that such a practice entails is slow.
We have been trying vagal nerve stimulation through the ear with a TENS machine. I don’t think it has helped, if anything it has caused a weird ‘buzzy’ sensation in the brain.
I am quite curious about stellate ganglion blocks. A recent study showed real benefits, noting the study was small and not placebo controlled.
We have had quite good results with acupuncture, although the benefits are fairly subtle
That’s because hearts require calcium, when calcium ion channels are closed they can’t get it, that where ldn helps alot!!
Years ago, when I was evaluating my adrenal and thyroid functions, I found that my heart rate did not increase as it should with exercise. I did not think to measure it again significantly later to see if it had decreased “normally” (for me). At the time, I attributed this “lack of sufficiently elevated heart rate” to low or deregulated adrenal (cortisol) production, and/or low or deregulated thyroid (T4, T3, or RT3) production or peripheral cell utilization problems. In response, I tried several combinations of different adrenal and thyroid medications, but none increased my heart rate, my temperature or my energy. Although my metabolism and hormones had a deficient response to exercise, supplementing the low hormones did not help. I was perplexed and moved on, realizing that there must be more to it than simply deregulated hormones.
This article seems to explain that my “inflexible” heart rate (in response to exercise) could have been due to ANS deregulation, wherein (in my words) the sympathetic phase was chronically activated – until my energy was exhausted (which would impede so many other systems that all need energy to operate properly). If I understand this article correctly, it seems to explain how the (sympathetic or chronically activated) ANS even remains over-activated during sleep – hindering proper or full detoxification, cellular repair and rejuvenation (all of which mostly take place during the para-sympathetic phase – in quality deep sleep – in a healthy person).
The ANS findings of this and previous CFS (related) research articles also seems to correlate strongly with my personal symptoms – both now and when I first got sick. I recall that when I first got clinically ill at age 30, one of my first complaints to my doctor was that; “I can just never seem to completely relax, so, the bed never feels good (anymore, or, the way it used to)”. However, my Doc (then and now) seemed to lack any clinical tests to evaluate sympathetic/para-sympathetic balance, let alone a means to control it.
Good research I think. If they can now figure out how to re-balance the sympathetic and para-sympathetic ANS phases in folks with CFS/Fibro/Long Covid, we could potentially have a very effective treatment at hand. Personally, I’d give my eye teeth for good, refreshing sleep. Thx for the great write-up Cort!
Hi Dave W.,
I agree with your presentation.
Back in 1979, low cortisol levels were actually the first indicator for me, pointing toward autonomic nervous system (ANS) involvement.
The only additional suggestion I’d make regarding hormones would be to also evaluate adrenal gland ACTH function.
Hello Sieglinde (and TY for your response).
Good suggestion! In my post above – for brevity – I did not mention that when I evaluated the role that deregulated hormones might play in my symptoms, part of that was an ACTH Exercise Challenge test. In this test, I had a blood test (in a hospital) that measured my resting cortisol and resting ACTH levels. Then, I did the most vigorous exercise that I could manage in the hospital (climbing up and down stairs) for 15 minutes. Then, I immediately had another blood test that (again) measured my cortisol and my ACTH. In response to the exercise stress, both should have been elevated significantly (I don’t recall the “normal” expected increase range) – but the increases in both my cortisol and ACTH were clearly abnormally low or blunted. This indicated that my pituitary did not increase its demand for cortisol sufficiently in response to exercise stress (by producing more ACTH as it should have), and (likely as a result) my adrenals did not increase cortisol production (as they should have). This told me that both the “P” and the “A” in the HPA axis were under-producing in response to stress. I did not know how to measure if the function of the “H” (hypothalamus) was also insufficient in response to exercise stress.
To reconcile these test results with ANS abnormalities, I wonder if the exhaustion of a perpetually over-activated para-sympathetic (fight or flight) ANS phase – could possibly include adrenal exhaustion – resulting in an inadequate immediate HPA response to exercise/stress (as my testing revealed)? And further, I wonder if the perpetually activated para-sympathetic phase may also prevent us from (properly) resting and rejuvenating (as we should)?
I’d like to see research that measured both ANS responses and hormone responses, to see if there are any correlating abnormalities, and possibly identify any cause and effect relationships between these two systems.
I’d also like to see ANS research measure abnormalities in terms of biochemical marker changes, rather than just changes in symptoms (such as heart rate variability) if that might be possible. This could possibly provide a clinical test for ANS function and balance (so Clinicians don’t go glassy-eyed when you suggest that your problems might be caused by imbalanced ANS responses). Specific ANS biochemical markers would also be easier to correlate with hormone levels (or other biochemical abnormalities associated with CFS/Fibro/Long Covid). Just food for thought. Good luck in your personal battle!
Hello Dave W,
I wanted to share my experience with two different ACTH stimulation tests that ultimately led to a clear diagnosis.
ACTH Test in the U.S. (Quest Diagnostics):
I arrived at the lab at 7:30 AM after fasting for 12 hours.
Blood was drawn at 7:45 AM and tested for baseline cortisol.
About 40 minutes later, I received what I believe was Cortrosyn (synthetic ACTH) via intramuscular injection.
I was then instructed: “Have some food of your choice, go shopping, and return after 4 hours.”
Four hours later, my second blood test showed a flat cortisol response, increasing only from 2 to 4 μg/dL — a result that is clearly abnormal and strongly suggests adrenal insufficiency.
ACTH Test in Germany:
Similar procedure — I arrived at 7:00 AM, had my blood drawn and tested.
This time, Cortrosyn was administered intravenously.
Within just 4 minutes, I collapsed.
I experienced severe hypoglycemic symptoms, including:
Cold sweats
Shakiness
Racing heart
Dizziness
This extreme reaction was a result of low cortisol levels, which couldn’t support blood sugar stability.
The cortisol deficit → led to unstable blood sugar → triggered more stress → which demanded more cortisol (that my body couldn’t produce) → causing a kind of cortisol crash or burnout.
I was immediately treated with glucose intravenous, which stabilized me. This clearly pointed to impaired gluconeogenesis, and likely a diminished glucagon (another kind of sluggish reacting hormone) response from the liver. The overactivation of my sympathetic nervous system was also evident — the classic fight-or-flight overdrive.
Post-Test Notes (U.S.): After the U.S. ACTH injection, I had a croissant and coffee with sugar, which helped replenish low glucose levels. However, it had no significant impact on cortisol levels, which remained flat — further confirming the diagnosis.
I’ve now been on 20 mg of hydrocortisone daily for the past 6 years, and I’m managing fairly well. With proper dosing and lifestyle adjustments, my symptoms are mostly under control.
Let me know if you find this different approach valuable, considering it engages both the sympathetic and parasympathetic branches of the autonomic nervous system.
My best for you,
Sieglinde
Before my hip replacement surgery a couple of years ago, I had to be cleared by a team in the cardiology lab. Because of my hip I had a chemical stress test instead of a physical one. The doctors ran the test three times because they could not get the heart rate high enough, but said there was nothing wrong with my heart. I think it was ANS dysfunction. During surgery my blood pressure plummeted to 60/30, and they gave me adrenaline, more ANS dysfunction I suppose.
Glad you managed to see Dr Ruhoy and you’re able to get another blog post out Cort! Much appreciated as ever. The upcoming blog on improving sleep sounds promising 🙌
Thanks, Cort. I believe we’re getting closer to the core of it.
As long suspected, it is the lower brain—particularly the hypothalamus—that commands the autonomic nervous system (ANS). It works in coordination with the brainstem (including the pons, midbrain, and medulla oblongata), the limbic system, and various hormones to regulate both the sympathetic and parasympathetic branches.
For this reason—and based on personal experience—I have long advocated for the initial evaluation to include both catecholamine testing and contrast-enhanced MRI.
Could it be damage to the vagus nerve caused by a virus that underlies the autonomic dysfunction and the imbalance between the sympathetic and parasympathetic systems? I’ve read that Covid can attack the vagus nerve and there seems to be some talk of damage to it being a factor in Long Covid. I experienced shooting pains which felt like nerve pain going up the left side of my neck when I had the mystery virus that triggered my ME years ago. Both times that I got Covid, I experienced the same thing.
If the vagus nerve has been damaged and isn’t functioning as it should then maybe the messaging system between the brain and body is messed up, causing some of the common ME symptoms? It would explain why just stimulating the vagus nerve doesn’t necessarily help.
”All signs point to the fact that too much exertion – particularly intense and longer duration physical (as well as mental exertion) – disrupts autonomic nervous system (ANS) functioning – in people with long COVID and ME/CFS/FM by putting them into a fight or flight (enhanced sympathetic nervous system) state.'(..)”
Yes Cort, the ANS again -:) We know this so long…. how can we fix this. Still, i think this is the cause of ME/CFS/POTS.
I have a very classic mix of ME/CFS symptoms – and no problems at all with the functioning of the autonomic nervous system. POTS isn’t required for an ME/CFS diagnosis. More, it is only a minority of patients who has POTS. Also, POTS exists without a co-existing ME/CFS. How is it then that the root cause of ME/CFS could be explained through a problem of the ANS?
From my personal perspective I experienced every neurological symptom as a direct result of the inflammation that was going on in my brain’s frontal lobe.
The whole experience of being incredibly ill without adequate support was very stressful and effected in a lot of fight or flight until I began to freeze at some point. The inflamed neurons didn’t make it any easier to handle my bouts of rage and intense fear, that would scratch paranoid mind states at the most extreme points.
I feel sorry for the ME/CFS community that we have entered the age of the hype of average quality neuro science, neurpsychiatry, neuropsychology (elsewhere also called neuro babble) that is not as in the old days done by direct research into the flesh but via the means of imaging techniques. These pictures are like a Rorschach test. Neuro “scientists” (elsewhere called “blobologists”) reading into it whatever they think works with their hypotheses.
I am fully serious when I say that I’d rather trust a tarot reader or an astrologist than work with whatever “cutting edge” neuro specialty there is nowadays.
Apologies, off topic!
Does anyone know the timeframe for the OMF’s itaconate shunt study?
https://www.omf.ngo/regulation-of-the-itaconate-shunt-in-me-cfs/
One of the most promising theories, in my opinion.