

Fluge and Mella seem to be working at almost lightning speed. Besides managing their huge Rituximab study (and all the sub-
studies within it) and the cyclophosphamide trial, they’re also carrying out large research studies.
For years, of course, some researchers and doctors championed the idea that problems with mitochondrial energy production were at the heart of ME/CFS. For many, though, the idea seemed almost too simple…too easy in a way. The body throws too many curves at us for something so obvious to be the cause. But it may be.
The work of Bob Naviaux of UCSD, Ron Davis of the Open Medicine Foundation, McGregor and Armstrong et. al. in Australia, Maureen Hanson at Cornell, and Fluge and Mella in Norway suggest that problems producing energy could, in fact, be causing the physical and mental fatigue in (ME/CFS).
Of course, it’s going to be complex. Exercise studies and other studies have suggested that the aerobic energy production pathways are severely blunted in a significant number of ME/CFS patients. Thus far, though, the metabolomics data suggests that the breakdown comes not in the aerobic energy production pathway but just before it.
Some key facts – such as I understand them.
- Key Factor in Glycolysis – Pyruvate – Pyruvate is produced by glycolysis and then gets broken down into acetyl-CoA for use in the mitochondria. When oxygen levels are low, the same process is used to produce ATP anaerobically. Anaerobic energy gets its bad rep because it produces toxins like lactate which at high levels cause pain and fatigue. All this occurs in the cell’s cytoplasm.
- Key Factor in Aerobic Energy Production – Acetyl-CoA – The first goal in aerobic energy production is to produce acetyl-CoA. This occurs in three ways: preferentially by converting pyruvate and/or by converting fatty acids or amino acids. The acetyl-CoA is then broken down further to produce ATP by a process called oxidative phosphorylation. All aerobic energy production occurs in the cell’s mitochondria.
The Study
At 302 patients (200 ME/CFS patients and 102 healthy controls) this was a nice big study. A different type of study than the recent metabolomic studies, it used a mass spectrometer to measure the levels of 20 amino acids involved in energy metabolism in the blood.
QT-RCT PCR was used to examine gene expression. Cells were also cultured, dropped in ME/CFS or healthy control’s serum (blood), tweaked with metabolic factors, and their lactate production and cellular respiration was measured.
Results
Amino Acid Results
Fluge and Mella did a simple but telling thing with the 20 amino acids by dividing them into one of three energy production pathways:
- Glycolytic Amino Acids – Amino acids used in the glycolysis pyruvate producing pathway which require PDH to be metabolized (alanine (Ala), cysteine (Cys), glycine (Gly), serine (Ser), and threonine (Thr))
- Aerobic Amino Acids – Amino acids that fuel aerobic energy production but which do not require PDH to be broken down((isoleucine (Ile), leucine (Leu), lysine (Lys), phenylalanine (Phe), tryptophan (Trp), and tyrosine (Tyr)) – mostly ketogenic amino acids
- Other Amino Acids – not found in the first category but which are essential to aerobic energy production (anaplerotic – methionine (Met), valine (Val), histidine (His), glutamine (Gln), glutamate (Glu), and proline (Pro), aspartate (Asp), (Asn + Asp = Asx))
Chronic Fatigue Syndrome (ME/CFS) – A Pyruvate Dehydrogenase Disease?
Fluge and Mella found no difference in the levels of the amino acids used in glycolysis – pyruvate production is fine – but reductions in the levels of the ketogenic amino acids used to power aerobic energy production.
Our cells much prefer using glucose to produce energy but the results and the Aussie study suggest that our cells are turning elsewhere.
Remember that our cells – use three different substrates (pyruvate, fatty acids and amino acids) to produce acetyl-CoA but they much prefer glucose. If pyruvate isn’t being broken down into acetyl-CoA, however, then our cells will turn to another energy source; in this case amino acids. The fact that virtually all the amino acids used to produce acetyl-CoA were depleted in the female ME/CFS patients suggested that their cells, starved of metabolized pyruvate, were turning to and using up amino acids to to produce energy.
Amino acids, unfortunately, are kind of like the body’s last straw for energy production. Our cells would much prefer to use glucose, but even fatty acids are a better source of energy than amino acids. In order for body to use amino acids it has remove an amino group (producing ammonia) and turn them into a sugar. (The fact ME/CFS patients appear to be turning to amino acids might suggest that they’re having problems with fatty acid metabolism as well.)
There’s another problem, though. If all this unused pyruvate is hanging around, it has to go somewhere. Unused pyruvate gets converted into lactate – a toxin responsible for much of the fatigue and pain associated with exercise. Lactate ultimately gets dumped into the blood stream. Fluge and Mella believe lactate accumulations are probably a key problem in ME/CFS as well. High lactate levels in ME/CFS patients’ brains have been found in several studies but reports on lactate accumulations in the blood are mixed.
Since pyruvate production doesn’t appear to be the problem, the problem must lie in the pyruvate dehydrogenase enzyme complex (PDH) which breaks down pyruvate to produce acetyl-CoA.
Or a Pyruvate Dehydrogenase Kinase Disease?
Fluge and Mella then asked why pyruvate dehydrogenase is not working in ME/CFS and may have found an answer in the increased gene expression (or activity) of the enzymes (PDH kinases (PDK)) that inhibit PDH. Rather encouragingly, they found that increased expression of one form of the PDK enzyme (PDK1) was associated with increased severity and longer duration patients.
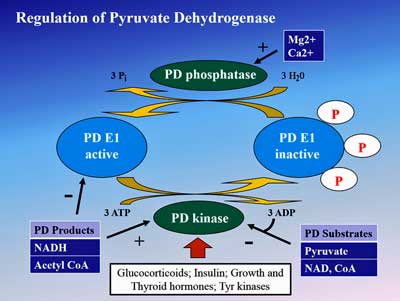
Fluge and Mella uncovered numerous irregularities that could be affecting the pyruvate dehydrogenase enzyme in ME/CFS
Digging deeper still they found increased gene expression of the transcription factor (PPAR) which upregulates PDH kinase in ME/CFS patients as well. Then they discovered that the gene expression levels of another enzyme (SIRT4) that limits pyruvate dehydrogenase production were increased as well.
Things were really humming along; Fluge and Mella’s findings suggested that every step in the chain needed to limit pyruvate dehydrogenase levels were activated in ME/CFS. Their consistently positive findings suggested that an inhibited PDH enzyme really may be a problem in ME/CFS.
Different Sexes – Different Compensatory Responses
The same problem (low acetyl-CoA levels associated with low PDH and PDK levels) was found in both sexes but men and women appeared to try to compensate for those reductions differently. While women demonstrated across the board depletions in the amino acids used to produce Acetyl-CoA, the depletions seen in men, on the other hand, were minor.
Fluge and Mella noted the small sample size of men (n=38) could have limited their ability to uncover significant differences but they also found increased levels of a substance in men called 3-Mhis which is indicative of protein breakdown or muscle atrophy in the men. (If the guys are wondering if their muscle atrophy might be due to more than lack of exercise, the increased levels of this substance could explain why. I’ve long felt that exercise results in a puffy look; could this be due to inflammation associated with muscle breakdown?)
One cautionary finding was that amino acid levels were not associated with disease severity; i.e. the more severely ill patients did not have lower amino acid levels than less severely ill patients. That was something of a surprise.
Cell Cultures
In one of the more fascinating aspects of this study, Fluge and Mella incubated (grew) muscle cells, put them in the serum from severely ill ME/CFS patients or healthy controls, and then analyzed their rates of mitochondrial respiration and lactate production (glycolysis).
They assessed the energy production of these cells under these different scenarios:
- Resting – cells in culture.
- Resting II – cells with glucose (ATP enhancer) added to the culture but with not extra energetic demands being placed on them.
- Under Anaerobic Strain – oligomycin is used to block ATP production causing the cells to rely strictly on anaerobic energy production.
Under Aerobic Strain – oligomycin plus CCCP – is used to produce a more severe blockade of energy production causing both anaerobic and aerobic energy production systems to work at maximum capacity.
- Rotenone and antimycin A – were administered to inhibit respiratory complex I and III, respectively, in order to assess nonmitochondrial oxygen consumption (subtracted as OCR background).
When the cells were not placed under any energetic demands they behaved the same whether they were in ME/CFS patients’ or controls’ blood.
When they forced the cells to exist solely upon anaerobic production, and when they put the cells under a severe energy strain, however, they found slightly higher rates of glycolysis in the cells exposed to ME/CFS patients’ serum. This suggested that something in ME/CFS patients’ blood was causing the cells to turn more to glycolysis for energy. Note, though, that the rate of glycolysis was only slightly higher.

The increased lactate production in cells put in ME/CFS patients serum suggested that minimal exercise could end up causing pain and fatigue
A further analysis indicated that when the cells in the ME/CFS serum were put under a severe energetic strain lactate production increased significantly. This time it was not a slight increase; the graph indicated huge increases in lactate production had occurred. That is what Fluge and Mella predicted what would happen when pyruvate dehydrogenase stopped working; the unused pyruvate would end up as lactate.
The surprise (at least to me) came when Fluge and Mella found that the ME/CFS patients’ serum did not cause the cells to reduce their oxygen consumption; instead, the cell in the ME/CFS patients serum actually increased their oxygen consumption both at baseline and when put under stress compared to the cells placed in the healthy controls’ serum.
Fluge and Mella suggested that the high oxygen consumption was a compensatory response to the problems with PDH they found. It may be that the mitochondria – missing their normal levels of acetyl-CoA – are sending out the message to get that glycolytic pathway moving to produce more pyruvate and hence more acetyl-CoA. No matter how much pyruvate it’s producing, though, it’s not enough for the mitochondria, because the pyruvate dehydrogenase enzyme has been turned off.
- The pattern of amino acid depletion seen suggests that women with ME/CFS are turning to amino acids to fuel energy production rather than the bodies preferred substrate, glucose.
- Possibly because of their lower sample size, men didn’t show the amino acid depletions the women did. They did, however, exhibit a significant increase in a substance which suggests they are breaking down muscle to fuel energy production
- Problems with an enzyme complex (PDH) which breaks down pryruvate so that it can be used to fuel the aerobic energy production process in the mitochondria are present
- Increased activity of the genes responsible for inhibiting the PDH enzyme complex is found in ME/CFS.
- Putting cells into the serum of severely ill ME/CFS patients caused the cells lactate levels to climb dramatically when the cells were put under energetic stress.
- Cells put into severe ME/CFS patients serum showed increased, not decreased oxygen consumption. Fluge and Mella believe the increase reflects an attempt to compensate for energy reductions associated with the PDH enzyme issues.
- A Stanford study, suggested, that the increased cellular activity appeared tied more to glycolysis than to mitochondrial energy production.
- Similar to what’s found in another severely fatiguing disease called biliary cirrhosis, Fluge and Mella believe that autoantibodies are attacking the metabolic pathways that regulate energy production in the body.
So far two attempts to compensate for the lack of mitochondrial energy production are possibly being made. The cell is upping its glycolytic activity (but notice that it was only slightly raised) and it’s breaking down amino acids for fuel. Fluge and Mella believe other attempts to compensate that we don’t know about may be being made as well.
The fascinating Stanford study will be covered more in a future blog. In any case, despite the twists and turns, the evidence thus far consistently points an arrow at the early part of the energy production process – glycolysis. Since glycolysis, and ultimately aerobic energy production mostly relies upon carbohydrates, the main metabolic problem in ME/CFS may involve the inability to properly metabolize carbohydrates. That, of course, would seem to make some sense given the problems many ME/CFS patients have dealing with carbs. Similarly, the depletion of ketogenic amino acids in the ME/CFS women suggests that a more ketogenic (i.e. fat and protein-rich, low carb) diet might be helpful.
We’re not at all done with the possible metabolic problems seen in ME/CFS. Fluge and Mella believe and Bob Naviaux’s recent study suggest that problems in fat metabolism in women – the third substrate used by the mitochondria – to produce energy also probably exist, and Chris Armstrong in an email stated that he thought that problems with both glycolysis and the Krebs cycle in the mitochondria exist.
PEM at the Cellular Lever
It’s kind of comforting to see the exertional problems show up even at the cellular level. As in the exercise studies, the cells in the ME/CFS patients’ blood needed to be put under strain to show significant differences. It turns out there’s an enormous difference (70-100 fold) between a cells at rest or being involved in strenuous exercise. That kind of leap in action, of course, leaves plenty of room for problems to show up if the energy production process is not working smoothly.
Next Up
Next up for Fluge and Mella: trying to determine: (a) what in the blood is affecting energy production; and (b) what is turning the pyruvate dehydrogenase enzyme off.
They believe their Rituximab success probably indicates that autoantibodies are attacking metabolic signaling pathways that are turning off the pyruvate dehydrogenase enzyme. They believe the same autoantibodies are also involved in the blood vessel issues found in ME/CFS.
They’re not grasping at straws. Dr. Julia Newton has found that an autoimmune disease called primary biliary cirrhosis (PBC) bears some startling similarities to ME/CFS. PBC is characterized by multiple autoantibodies that impair mitochondrial functioning and target the PDH enzyme complex believed to play a role in ME/CFS. Besides the enormous fatigue they experience, PBC patients also display increased sympathetic nervous system activity, and problems with orthostatic intolerance and cognition are found.
In fact, last year Julia Newton, stating that she hopes the results will be similar to those in ME/CFS, began a Rituximab trial in PBC patients.
Coming up next on HR – more studies are flooding in. Coming up shortly on HR, a look at the Aussie and Stanford studies.
- Learn more about ketogenic diets in Health Rising’s Diet Resource section
- Check out more blogs on metabolomics in chronic fatigue syndrome (ME/CFS)
Health Rising’s Big (Little) Donation Drive is Successful! – Thanks to over 214 people who donated we have met (and exceeded) our target :). ( I, of course, am much relieved :)) You’ve ensured that Health Rising will continue to bring you the latest news on ME/CFS and FM research and treatment into what appears to be a very exciting 2017.
Thanks to all who have supported HR this year including Dennis Sylvain for providing research papers, Marilyn for her rapid editing help :), an anonymous friend for her support in many areas, to Stavya for his impeccable work supporting the technical side of HR, to Glori for her artwork, to the Simmaron Research Foundation and Prohealth for their financial support, and to everyone who has provided helpful comments.







This is all getting so interesting!!
I’ve been saying for a long time that the lactic acid production was a key problem, the Norwegian research is very, very hopeful!
I think that in any mitochondrial disease, where oxidative phosphorylation is deficient, the backlog of lactate, pyruvate, and acetyl CoA metabolism may be a significant contributor to the disease process. These compounds don’t just sit passively around the cytosol. Does anyone know, offhand, if there is evidence of changes to the actin, tubulin, and myofibril structures in ME? Are there losses of function in peripheral neurons with long axons? Sorry to ask, but I am a little buried in unread literature. The first disease I started studying was Friedreich’s Ataxia, and now I am looking around for analogous processes in other mitochondrial diseases.
Thanks, Cort, for not only explaining these new Fluge and Mella results, but how the energy processes they point to can mesh with findings of other recent studies.
Although it is good to hear that specific abnormal processes are being observed (second-hand through biochemical evidence), it is also good to hear that some cellular processes appear to be normal! Maybe us patients are still mostly human after all. 😉
Thank you Fluge and Mella, and whoever funds your research!
I try to use the small amount of strength I have each day to make the healthiest food I possibly can – I live in a remote area and there are no ‘stores’ I can go to to purchase unprocessed food so if we are going to eat such, I have to make it. So, I make it my priority and most mornings I am in the kitchen attempting to make enough food for the day and hopefully enough to last another day or two, and without fail by 30 to 40 minutes into it, I begin to get such searing burning pain in the muscles across my back. Of course I have to press on to finish my tasks and the pain levels just increase and increase. No doubt the lactate levels at those times would register very high indeed.
This explains again why Amino Acid testing and replacement of the low ones gave me back 80% improvement in function, especially neurologically. I was only diagnosed with FM, but have long suspected I also have ME/CFS. Interesting enough, about 10 years ago, a Rheumatologist told me autoimmune was showing up in my lab tests, but she could not pinpoint why!
I noted years ago, that if I added bread to a meal, I would lose most of my energy. What great information in this article Cort. It has answered a lot of the questions I’ve had throughout the years. For those who are interested in AMINO ACID TESTING, google: http://www.doctorsdata.com for more info. It’s only a 24 hour urine collection, but your doctor has to contact them for prior arrangements. They provide an extensive report which also includes the exact prescription that can then be filled by a compounding pharmacy. Amino Acid replacement has given me my life back! I cannot recommend it enough!!
Thanks Kathy, very interesting. Our main problem is a lack of doctors that would.
Lactic acid is associated with fatigue and pain in muscles, but is not the primary cause, at least in normal people. Its a sign, not causal. However if there are local concentrations that are very high then it might activate the acid sensors. Lactic acid actually increases capacity for aerobic respiration in healthy people by forcing a local alteration in the oxygen dissociation curve in red blood cells, which then dump more oxygen. Since the normal situation for high lactate, that is non-ME patients and non-severe heart patients, is due to low oxygen and high carbon dioxide, the local oxygen level restores energy production. However local carbon dioxide can cause acid burn. Its a failure to clear carbon dioxide.
I was talking about energy blocks and amino acid depletion since about 1999. However I thought the block was at aconitase and could not get enough data to support that. Back then I was sometimes called the alanine guy, as I recommended alanine to people. It did help me a little. My model at the time was mostly likely wrong though.
In the 90s a bunch of Australian docs tested their patients on different protein diets, and empirically decided that about 1.5g/kg of body weight was the ideal protein amount. This is about half again what is normally recommended, a little higher than a diabetic diet, but not quite a high protein diet. I don’t think this was ever published, though I could be wrong.
There is something wrong or incomplete with this article’s explanation. Its bugging me. I still have too much fog to really figure it out though. In part this is because oxygen utilization is typically more or less in lock-step with ATP production, and hence energy production. I think this might tie in with what is happening with other mitochondrial metabolites and byproducts, and I hope to investigate that if I get my brain even half working for a few hours. If oxygen utilization is high I have to ask where is it going?
Thanks Alex for all the info. It’s really confusing to me to me.
I realize that in the presence of oxygen lactate gets turned back into pyruvate. (The increased oxygen consumption found in the cells cultured with ME/CFS patients serum suggests that oxygen levels (in the mitochondria) are fine.
I thought that oxygen utilization must be in step with ATP production but I wasn’t sure. I haven’t read the Stanford study in detail but it suggests that the ATP production in ME/CFS is actually high but they mention increased glycolysis as wel.
Could it be that there is a problem with pyruvate breakdown – and that glycolysis is going bananas in an attempt to compensate – and ATP production is high, but somewhere along the line the problems with pyruvate breakdown are creating issues?
Perhaps there is a problem with lactate accumulations even in the midst of oxygen? (Newton had that idea I don’t think that turned out for her, though.)
Since over a year I started asking myself with every symptom: is it really an undesirable effect of the disease? Or could it be the backside of a healing mechanism? I compare it with not-to-high fever, sweating, fatigue, muscle soreness and pain in flue. They are not the disease, the virus taking over your healthy cells is. The fever helps killing it, sweating helps removing toxins and fine-tuning temperature, fatigue, muscle soreness and pain help in deactivating the patient so that he spares his resources to recovery.
One thing what strikes me is this: “Fluge and Mella’s findings suggested that EVERY step in the chain needed to limit pyruvate dehydrogenase levels were activated in ME/CFS.”
Now, one or two things going bad, you have a higher chance of getting sick. Many things going bad, you ‘ll be very severely ill. But ALL things going bad? That may well be a VERY determined body that ABSOLUTELY wants to enable this state. I observed it several times with my own body: at my worst episodes my body seamed to go through extraordinary length to keep generating my symptoms. The more I tried to undo them, the more my body seamed to go into a mad frenzy to get those symptoms back.
I suspect there is a small chance that the body wants to convert as much as possible protein to glucose. After all, if you did eat it (easy to test) and did take up a decent part of it (not removed in toilet, easy to test) then it must go somewhere. If it is not stored somewhere, it must be converted to something else. For example to glucose at max speed. Why? Maybe because:
* pyruvate is ketogenic and made out of glucose. ketogenic diets seam to help some patients and could remove ROS (explained in another post).
* massive consumption of amino acids should produce high loads of uric acid. Since going moderate carbohydrate diet, I eat way more protein then before, but uric acid (despite familiar weakness) is not even a slight problem in my blood analysis. Uric acid however is a good anti-oxidant as well. Is it consumed by ROS in my body? Consuming as few carbohydrates as possible (Palleo diet) allows your body to convert far more amino-acids to glucose and pyruvate producing more anti-oxidant. Doing so as inefficient as possible (anaerobic) allows to produce even more. Throwing ATP out of the cells (observation Naviaux) instead of using it allows it to convert at even a higher rate.
Concerning the odd increased oxygen consumption: it must go somewhere, it can’t just disappear. Maybe it goes to extreme amounts of peroxide production? That is part of the Cell Defence Reaction too. Even in healthy people, peroxide production ramps up very fast at prolonged high levels of exertion. Now combine it with the previous: it appears that only at overexertion metabolism goes really bad. So it could produce extreme amounts of anti-oxidant (pyruvate and uric acid) at nearly the exact time of overexertion and most at those cells overexerting.
It may be likely that both supposed extreme ROS production and anti-oxidant production at the site of said ROS production are actually badly needed. For example as last-line pathogen defense system. Fluge and Mella found the white blood cells severely weakened as well. So a strong CDR may be very much needed to suppress opportunistic pathogens like EBV, Borrelia, … Producing so much anti-oxdiant at the same place as the ROS may protect the producing cell itself against the ROS. It could not get enough anti-oxidant out off the blood fast enough at such a respiratory burst. As ROS is a signaling molecule triggering more CDR and ROS, it can go quickly to overwhelming ROS production (onset PEM, which I find to go extremely fast once it starts?).
Next to toughening the cell, producing much ROS to kill pathogens out off the cell, triggering mast cells to go boom… lactate is used as a mild anti-bacterial agent in several medical and industrial applications as well. The total picture may reveal a Mexican standoff between opportunistic pathogens, a strongly weakened immune system and a body going all-in in CDR to survive this condition. As such standoff is kind a slow-motion war, it may not be recognized as the dire emergency it is by measuring classic infection markers.
Thanks Dejurgen for your interesting ideas:)
I was diagnosed with severe f/m years ago and over the years notice a definite fatigue and cognition decline with the fatigue. I also noticed it takes a full three days after any particular exertion for the full weight of the pain to kick in with all of the attendant stiffness and headaches.
For the first time I feel like there’s going to be an effective treatment in the next 5 to 10 years – one that won’t potentially kill me.
10 years is far too long for some of us.
The way I think this is going to go is bit by bit. First something will be find that helps quite a bit; then another thing will be found – then another thing and like that – so hopefully nobody has to wait ten years for relief but can start getting some relief fairly soon.
Naviaux is going to do a small clinical trial in the first part of next year I believe.
What is Naviaux going to be testing in his small clinical trial? Does he have treatment ideas?
I believe its a drug called Suramin – treats Sleeping Sickness believe it or not.
Here’s something I found odd, or a rather big coincidence. I looked up PBC. Looked at Mayo’s site, which I would think would be the best source for how conservative medicine defines/treats it.
The list of drugs, for various symptoms/treatment, is remarkably similar to things that have turned into major parts of various lyme/bartonella, mold, ME/CFS (and SIBO, etc etc) protocols. A number, they are not sure what the mechanism that helps is.
This seems strange to me, like some kind of entirely different tracks but the same structure coming up around it. I really have never seen Cholestryamine, Rifampin, Naltrexone, etc in the treatment of any other disease—except those they are explicitly used for (infections, cholestrerol, addiction).
The Mayo page even ends with something my doctor just mentioned to me, having nothing to do with PBC, the depletion of those specific vitamins in our ME/CFS-mold-lyme spectrum.
Maybe this is silly, hoping someone more knowledgeable would consider.
Interesting Michael,
The results of the PBC Rituximab trial are going to be fascinating.
Michael, at the very end of your post which very specific vitamins are you referring to?
I just posted a thread in the general forum you may be interested in. I don’t know anymore than anyone else here (certainly much less!) but we need many heads to solve complex problems. I listed a number of studies that consider autoimmune diseases and acetylcholine. Missed PBC, but guess what found one just now: https://www.ncbi.nlm.nih.gov/pubmed/1692155
Please share your thought on the forum if you have a moment.
I want to share the three major interventions that have helped my Chronic Fatigue Syndrome. They are not directly related to the content of this current issue of the blog but who knows they may be related to the research described here in the long run. The first is that I had an overnight positive response to an anti-inflammatory that is herbal. It is called Kaprex and consists of a mixture of Olive leaf extract, rosemary extract and one compound from hops. It functions as a Cox 2 inhibitor, like Celebrex. Literally overnight at the minimal dose I experienced a decrease in pain and increase in energy and an increase in my mood and motivation. The second thing that has helped keep the brain fog lessened is taking 4 g of salt a day. I have mild hypertension and that huge amount of salt intake has had no negative effects on my blood pressure. Taking that does regularly keeps my brain fog at bay. When I begin to feel a bit foggy and have been doing a very small amount of exercise or have been perspiring I frequently need to take an additional 1 to 2 salt tablets. The most recent intervention is getting 10 fecal microbiota transplants at a branch of the Taymont Clinic in Nassau in the Bahamas. Getting the FMT’s was a minimally unpleasant procedure and I noticed about three days after finishing the course of the 10 of them that my brain fog had decreased dramatically along with improvement in my memory, concentration and overall cognition. I first developed fibromyalgia a year after having bacterial pneumonia and a months worth of antibiotics. Several years after that, following spine surgery at Stanford Hospital I had an undiagnosed epidural hematoma which left me partly paralyzed and develop Clostridium difficile which went undiagnosed for the following 3 1/2 years. After nine months of three antibiotics the infection ultimately only responded to a fecal microbiome transplant. I had had another episode of Clostridium difficile several years later which rapidly responded to vancomycin but obviously wiped out my microbiome. Since getting the 10 FMTs I’ve also noticed that my bowel movements which used to be usually soft and poorly formed have become much more regular and much more fully formed. After the 10 FMT’s when I noticed that I was becoming more depressed again I began to take Strattera, which I had taken a year before to give me energy. This time it is taking a much lower dose of Strattera to give me the necessary energy and improve my mood than I did before. I had no adverse reactions to the 10 FMT’s other than the expectable slight decrease in energy and increased need for sleep after the first 3 to 4 FMTs. My micro biome is being monitored with monthly specimens to analyze my micro biome at the Open Medicine Institute.
Thanks for sharing that Deborah and congratulations. On a scale of 1-10 with 10 being healthy, how would you say you are doing now?
Deborah, is the clinic expensive? why did you choose Bahamas? I’ve been wanting to do FMTs but am afraid to do it by myself with a donor outside a clinic.
2 years ago, I had 20 DIY at home FMT’s and I noticed a large benefit after the first couple. I felt like finally I was on the road to being “cured” I felt that much better. However, I screwed everything up by taking too much fiber and pre-biotics in an attempt to support my FMT. I really shouldn’t have changed my diet or supplements at all. Since then, my FMT donor hasn’t had normal BMs or gut so I haven’t been able to repeat the FMTs.
hi! i have so many questions about FMt! straterra! salt! would you mind giving me some more information?
An increased need for sleep? Isnt that a good thing?
With CFS, the primary common sign (per Dr. Teitelbaum, MD) is the inability to (ever) get a good night’s sleep and wake energetic and refreshed.
After a year recovering from a severe bout of CFS, down to a 1 on the 0-10 CFS Energy Scale (triggered by a Flu shot!), I am up to 4 to 5.5 hrs of sleep a night from 3.5., and a 4 on the Energy Scale. The rest of the time, I am too awake to sleep, but too tired to read more than a few pages before my mind drifts and I can’t stay focused.
With the current need for more sleep than 3.5 hrs/nt, I feel less ability to focus and more forgetful (what did I come in this room for?).
Does anyone else here find that, too?
Hi Susan, memory problems come with our illnesses. I found that lack of quality sleep plays a big roll. I’m on prescription sleep meds. that are working well for me and I hate to rock the boat by trying something new, but if that was not the case, I would love to try a natural one. The one I’d like to try is FIBRO SLEEP, available at http://www.prohealth.com Hope this helps!
Susan, thanks for the product tip! I bookmaIrked it.
I’ve padded my mattress with a down comforter so there’s less pressure on my body, therefore better blood circulation. Got the idea after staying at a Ritz hotel and having a fantastic nights’ sleep on a bed that felt like a cloud. Thinking about going back to a motionless waterbed.
I’ve been making a drink before bed that helps the quality of my sleep: https://www.davidwolfe.com/turmeric-coconut-milk-better-sleep-digestion/
Sometimes I just drink an organic coconut water instead, I like King’s Island brand.
Interesting article on why this works; and sugar. What we need during the day to fight POTS as well!
http://butterbeliever.com/how-to-fall-back-asleep/
When
Hi Susan, I too had a flu shot and wosrened from a 7/10 to a 1/10. Worst I’ve felt in years.
Anyway as for sleep, I also used to have trouble sleeping, but now listen to audiobooks where the reader talks in a monotone, but the story is interesting enough to follow. that combination keeps you listening, and seems for some people to shut down the brains internal chatter. i.e. the narrator of the audiobook takes over this chatter. I have found that the narrator has to be quite monotone though, with very little dynamic in the voice. so listen to the samples of different narrators first. (I find the deeper male voices more sleepy)
Theres also a podcast called ‘Sleep With Me’ where a guy with an American accent who has that hippy (almost a stoner accent) talks in a monotone voice about things interesting enough to start listing to, but he soon goes way off topic. The brian follows a couple of these tangents of topic change, but that sleepy monotoned voice soon has people drifting into lala-land. Note the first 20 mins of that podcast is a little more upbeat, then after that he starts elongating his words and virtually talking nonsense. Which is a good thing as the brian becomes nonsensical at sleep anyway. Some people say ‘Sleep With Me’ is brilliant for insomnia, and some people say it doesn’t work for them. I’m lucky it worked for me. The best thing is its free! Some of his podcasts are better than others so try a few of them.
Be aware though not to have screens from computers or phones in bed as they keep the brian active. To avoid that I bought an extension cord for my headphones so my phone is out of reach. I set the volume first and the headphones. (also set the sleep timer so it doesn’t play all night haha)
Thank you for this interesting article, Cort. For many years I have had the searing pain across my back that Kathy refers to and her response has made me realise that it is due to ME and lactic acid rather than the problems I otherwise have with my back. Unfortunately, my doctor is of the biopsychosocial persuation and no amount of enlightenment on my part will make any difference to the overall picture of my condition to her. Interesting for me, though.
Thanks Cort for another useful article. There is one point I don’t understand in your explanation. You say:
‘Key Factor in Glycolysis – Pyruvate – In the presence of oxygen, it turns glucose, a carbohydrate, into pyruvate. Pyruvate then gets broken down into acetyl-CoA for use in the mitochondria. When oxygen levels are low, the same process is used to produce ATP anaerobically.’
But as I understand it,glycolysis is entirely anaerobic. Oxygen only comes in as part of the aerobic respiration process later in the mitochondria.
Can you clarify.
Big, big sigh here.
Glycolysis plays an important role in aerobic energy production but it, itself is entirely anaerobic. That’s really disappointing because I had hoped that some of the increased oxygen consumption was coming from bumped up glycolytic action. I should have realized that that’s not true because Fluge and Mella found increased lactate production and I believe that lactate is only produced when not enough oxygen is present.
That means, though, that the increased respiration found in the cells in the ME/CFS serum was entirely mitochondrial. That is such a head-scratcher for me; if the mitochondria are turning to amino acids – not a great substrate for them – why are they doing so well? Are mitochondria not the issue at all? Is the entire problem due to pyruvate being turned into lactate which then causes fatigue and pain? We’ll see about the lactate findings. Reports on lactate have been up and down.
I think there are still some twists and turns to come…..there usually are in medicine.
This issue is about the NAD/NADH ratio. If my brain were working better I would be writing this up in detail. Increased oxygen utilization is tied to increased NAD conversion from NADH, but when oxygen is short then pyruvate to lactate substitutes for increased NAD production. We suspect from other ME research that there might be something wrong with the NAD+NADH pool. Its potentially a substrate shortage though there may be other problems. The body switches from pyruvate-lactate to electron-transport-chain-oxygen, as a source of NAD, and you need NAD or glycolysis will stop. No glycolysis probably means rapid death.
Something else I am thinking about is the concept of uncoupling, where energy production can become disconnected from energy utilization for normal circumstances. In other words there may be a loop that is chewing up energy and oxygen without producing useful output.
Alex, that’s interesting what you say about NAD and NADH ratio. I can pinpoint my CFS worsening when I used up too much NAD+ and starting getting lactic acid symptoms. Do you know of any anecdotal reports (perhaps on phoenix rising) of anyone taking massive doses of NAD and their outcome?
I would be dubious about massive doses. There is some talk of moderate doses over time helping, but I don’t know if lots of patients have improved. I do vaguely recall there was a formal study on NADH though. I don’t have a reference handy.
Cort, does this mean there is also acidose?
I think we have increased acidity during exercise, with the potential for increased alkalinity during rest. Is that what you were referring to? In other words we are often alkaline, not acidic, but that rapidly changes when we do too much. It also takes a long time to go back to alkaline.
I suffered with ME for eight years. I was then free of it for 12 years. Now i have an autoimmune disease which i at first thoght was ME but nearly killed me. This is not getting better, but i have had riduximab and that worked for me. Pain and severve tiredness are my constant companions. I strive to live for a day i can be normal again.
Whether ME/CFS is associated with autoimmune diseases is a question that I don’t think studies have answered but it’s certainly an important one. How did you recover the first time?
For me this study makes more sense than any other I have read about. I have dreadful problems with low blood sugar after exertion and have to eat spoonsful of honey. I have long suspected that there is a supply problem that demands extra compensatory glucose.
Thank you so much Cort for all your hard work. If it wasn’t for your excellent information I would have lost hope by now. I am about to initiate a regular contribution.
Thanks Christine for your support!
Excellent article, Cort. Your ability to absorb and translate these scientific studies into something I can understand is amazing and so helpful. This study makes sense to me, and coincidentally, I have been feeling much better recently following a low carb high protein diet. Or maybe not coincidentally?
Cort, your posts are so well informed and so encouraging- I hang on to them!
Thank you so much for such excellent work.
Thanks, Ruth 🙂
This is all very interesting, but I’d like to make the point that all these findings are secondary – the key fact is that excessive interleukin production is causing cells to ‘hibernate’
This, after all, is one of the key roles of interleukins. This is to deter cells from becoming ‘factories’ for viruses
we know that suppressing interleukin producing B cells helps 2/3 of ME patients.
The key question then is, why are B cells over producing interleukins ?
lets not lose focus on the root of the problem
Can you refer to any study that says “the key fact is excessive interleukin production is causing cells to ‘hibernate’?”
I understand that is one hypothesis of how the rutixmab works but haven’t seen that fact shown in any studies.
Here is an article written by Fluge/Mella/Tronstad them self about the recent study:
http://kavlifondet.no/2016/12/new-study-on-pathological-mechanisms-in-me-from-bergen-research-group/
I’ve long forgotten my pathophysiology on glycosis, energy production, excessive lactate etc.
However, I remember enough to make most sense of your article.
This explanation makes a lot of sense and I’ve long thought there is an issue with max Vo2 levels after exercise and the build-up of lactate. Someone mentioned acidosis and lactate is acidic.
Dysfunction in energy production anywhere along it’s pathway will cause havoc. There was a mention of increased interlukins as being the “root” of the problem-causing the cells to hibernate. My science knowledge is not high enough to completely understand what’s going on but both theories make sense.
It’s sort of like the “big bang” theory-what started the initial Bang!
Thanks so much for shining a light down this black tunnel of ME/CFS, Cort.
I hope the funding gods at NIH keep up with the research of others. Hats of to the Norwegians.
Can anybody answer for me if it might be worthwhile to increase protein intake or is the body already quickly maxed out on how much it can process? Thx.
Hi Greg. My Naturopath Dr. had me increase my protein intake that did not help me much, then he told me to use a good quality digestive enzyme, Panplex 2 phase by Integrative Therapeutics, which was extremely beneficial for IBS. He decided after all that to have my Amino Acids tested and the essential ones that come only from food were still rock bottom. If you go back up to the 5th comment, I explain more about the testing and prescription.
Chris Armstrong recommended good digestive enzymes if you’re on a ketogenic diet.
Out of interest, what were the animo acids you were deficient in?
As I understand it, the important ones to supplement should be the ketogenic animo acids, which break down into acetyl-CoA.
Of course supplementing these doesn’t solve the root issue – or deal with the pain generated with lactic acid. However, if our bodies are truly using animo acids as the main metabolic method then perhaps it’s not a bad idea to try supplementing for more energy?
I’ve been dealing with chronic fatigue for about 18 years now with varying success. Lately, another family member was put on a low carb/mod protein/high fat diet at the advice of his cardiologist. I decided I would do it as well.
Almost 3 months in, I’m well into ketosis (burning fat for fuel rather than sugar) and have lost more than 25 lbs due entirely to the change in eating (another 15 from exercise). I still have PEM after strenuous activity, although it is considerably blunted. I very rarely take naps any more.
Is it the solution? Certainly not the only solution, but for me it is a piece of the puzzle that has been confounding me for quite a while.
It’s been helpful (although not the answer) for me as well.
I tried first a normal high protein low carb diet for about a year but didn’t feel any better. I stopped for 6 months. However not one to give up I thought why not try plant based proteins and fats instead. I found that theres plenty of plant proteins and their isolates on the market and I was impressed to see, even combinations of different types that have all the amino acids needed. Also this time instead of using animal fats I used flaxseed oil (high in omega 3) and also increased my plant intake, including avocado and raw macadamia nuts. And the effects were really good. much better than the animal protein diet. (The plant protein/fat diet was over 12 months also, and continues)
I have since read studies that animal proteins cause inflammation in the lining of blood vessels hence another possible mechanism to why animal products are linked to heart disease. I see that Fluge and Mella have also hypothesised that the endothelial cells appear inflamed and may be being attacked by an antibody. Could it be then, that the body is getting confused by mistakingly targeting our own body’s endothelial cell proteins as foreign animal proteins? i.e. Are animal proteins adding fuel to the fire. Are they causing or aiding an immune distraction?
I say this because the difference between the two diets high animal protein/fat and high plant protein/fat diets the latter was a significantly noticeable improvement. Yet the dietary intake levels were the same.
Note neither diet was a cure, the plant one was the only one that noticeably eased symptoms.
Also more good news… Just late last year I decided to take the drug Sildenafil (Viagra) and within 3 weeks had dramatic improvement in energy output. I did this after reading about Fluge and Mella’s patent on Iososorbide Mononitrate, (because I couldn’t get my hands on it, I had to settle for the nitrate Sildenafil).
I told my specialist who was impressed enough that she decided to try me on Isosorbide Mononitrate, unfortunatly that didn’t work for me. So I will be trying Sildenafil again. (I will update you when I do)
The dosage of sildenafil for me that worked was slightly higher than the recommended dose of 100mg per day.
Dosage: 30mg 4 times per day. (Try less first)
Best try the recommended does first. Also don’e expect a miracle. I still wasn’t cured on it. just felt a lot better and it could have been pure coincidence that I was in remission.
NOTE: long term use of Sildenafil can cause hearing problems and other side effects. check with a doctor on these first.
No matter what though I’m still sticking to the plant protein/fats diet. I still include plenty of whole plants, fruits, nuts, legumes, seeds etc. No refined foods whatsoever. I guess its virtually a animal protein free Paleo vegan diet but a very healthy one.
Can This occure when on T4 (Levaxin)?
I cannot understand much of what you have written here, because I don’t understand glycolysis.
I’m sure that you’re not alone 🙂
Many thanks for the fascinating article, Cort. You mention generally which amino acids were depleted: “reductions in the levels of the ketogenic amino acids used to power aerobic energy production” and “virtually all the amino acids used to produce acetyl-CoA were depleted in the female ME/CFS patients”. Is there a list/table of specifically which amino acids these are? (> possible supplementation). Sorry if I’ve missed something.
All the 6 amino acids in category II were significantly reduced in nonfasting ME/CFS patients compared with nonfasting healthy controls, with P values of less than 0.001 for Ile, Leu, Phe, and Tyr and P values of 0.001 and 0.009 for Lys and Trp, respectively (Table 1).
Unfortunately, George, it only applied to women! Men did not have reduced amino acid values; instead they had increased Methyl-his or something like levels which suggested they might be breaking down their muscles for proteins.
Amazing. Such an interesting article and findings. My 15 year old daughter has CFS – I tried to explain the gist of this article (without fully understanding it myself) and she understood it straightaway. Thank you so much for this information!
🙂
This is really interesting.
I don’t have a CFS/ME diagnosis but have some other conditions and stubborn and debilitating fatigue that doesn’t respond to the treatments for those other conditions.
I recently had an organic acids urine screening done, and both my carbohydrate metabolism and fatty oxidation seem to be impaired, and also some wonky results in my Krebs cycle.
Though I had low pyruvate levels (and high lactate) so I am not sure what’s going on there. My brain is too frazzled to get my head around it all and put the pieces together (along with some genome results)
I learned previously through trial and error that amino powder drinks and protein shakes(the ones bodybuilders and gym fanatics use) make me feel a bit better, and that might be explained by my apparently difficulty in using carbs and fats as an energy source.
It sounds like there could be potential in these paths of research and I very much hope that further investigations will help lead to better treatment options for those diagnosed with CFS. My heart and thanks go out to the dedicated researchers who are taking it seriously and putting in their time and work.
It makes such sense that chronic fatigue could sometimes be caused by metabolic or mitochondrial dysfunction, yet so often patients are told there’s nothing wrong because their test results are “normal” because the doctors don’t test for these things, only standard blood tests for FBC, thyroid, liver, kidneys, anemia etc. I’m sure more patients would have abnormal results (and clues as to what is going on and therefore what might help) if we were tested for these things. Why can’t it be standard that after normal bloods, patients presenting with persistent serious fatigue aren’t tested for metabolic and mito stuff, given that energy processes happen via that? Surely that is only logical!
Energy problems? Let’s test how your body is processing input and generating energy.
Thanks Cort. For many years I suspected most strongly a virus as the cause of ME for me. My thinking has changed in recent years as I have watched my children grow and exhibit similar prodromal issues to me such as mild orthostatic hypotension, PEM and digestive issues. My sense now is it is more genetic and metabolic issues and related to other areas such as hypoxia, aspergers, Parkinson’s, i.e. neurological. That is not to say viruses are not involved but rather that latent viruses are enabled in a hypometabolic state. Interestingly, there was research done in the 1960s on the interaction of gut bacteria in the process of activating pyurvate dehydrogenase. Also of note, the most common treatments for people with severe genetic pyurvate dehydrogenase deficiency at birth are ketogenic diets, thiamine and buffering agents such as sodium bicarbonate. I write this on a now rare visit to the Rocky mountains. It intrigues me how altitude sickness (hypoxia) seems to trigger, mimic or excaserbate my ME symptoms very closely. Altitude sickness/hypoxia are well known inhibitors of pyurvate dehydrogenase…
Interesting stuff Simon…I like this idea – My sense now is it is more genetic and metabolic issues and related to other areas such as hypoxia, aspergers, Parkinson’s, i.e. neurological.
and this one: That is not to say viruses are not involved but rather that latent viruses are enabled in a hypometabolic state.
The best health I’ve experienced since becoming ill with me/cfs 15 years ago was while undergoing fertility treatments. I noticed this 2010 study that said the remission women with MS may experience during pregnancy may be due to a REDUCTION of pyruvate kinase activity, I.e. The opposite of what is going on in mecfs: http://kellogg.umich.edu/news/20100615_PETTY.html
Interesting to see those pieces maybe fitting together.
Very interesting! As I understand it a reduction in the pyruvate kinases – which are inhibiting pyruvate dehydrogenasae – might be a good thing in ME/CFS. We know that some mothers with ME/CFS do get better during some stages of pregnancy.
My concern is the lack of researchers explanation on how their hypothesis falls within the historic epidemiology of this illness,considering the fact that this illness is associated with various hotspot outbreaks and relative short incubation period of symptoms.
What is causing the sudden initial down regulation of the metabolic pathways?
Lipkin’s research alludes to a change after 3yrs which could explain what is now being investigated. I am curious if their findings hold true to patients within 1-3yr period?
Good question! T just talked with a group which thinks they have the answer to that. They have a very different approach.
The hypothesis that has been around a long time to explain this is that severe viral infections, or other infections or injury, can trigger the (metabolic) issues. They are possibly NORMAL in severe infection, we just don’t know for sure. However this becomes stuck.
I am keen to see research on all the alternative explanations. We need to start ruling them out, until we are left with only one explanation that works. Arthur Conan Doyle (Sherlock Holmes) could have been talking about us.
It seems all very fascinating although I understand only a small part.
I cant wait for the next results – it´s horrible how inpatient I am.
Can we already expect next year some publications from these teams which study metabolic problems or will it take again longer?
many patients have some positive experiences with ketogenic/paleo diet.
But regarding this research is it not more increased protein intake than decreased carbs intake what helps patients?
I tried ketogenic diet and I felt energy increase already after few days. I followed very well this diet but after 4 months I started to feel hypoglycemic so I had to increase my sugar intake (but it was still less than before my ketogenic diet). Now it´s almost 1 year that I am eating more sugar but my energy level is the same. So I am thinking that maybe with ketogenic diet it´s more increased protein intake what really counts. But maybe I understand it completly wrong.
Really impatient to know more 🙂
Come one guys you are very close to the answer back in 1994 they found Glycolysis abnormalities in fibromyalgia, they saw increased pyruvate and decreased lactate production in FM. The body isn’t doign this by mistake the body is producing energy from lactic acid, painful long term but its keeping you alive, I belive i have cracked the root cause and my wife is in less pain and more energy, just refining the protocol, the answers they guys just track the pathways.
Can you give us a hint as to your protocol?? I’d like to give it a try.
Pam drop me an email at s@unders.com.au and ill give you some information, if you give me your back story that would help and also if you know your blood pressure (before any medication)
Yes, I too would like to know what helped your wife.
I am going through an incredible stressful time right now and the last time this happened I lost weight down to 84 pounds.
I need to prevent this somehow.
Kathryn drop me an email at s@unders.com.au and ill give you some information, if you give me your back story that would help and also if you know your blood pressure (before any medication)
I would be careful about going on a high fat, high protein,low carb diet. I couldn’t digest this diet and ended up in serious trouble (bed bound, severe weight loss, unable to recover from activities) for several years. i am now on a high sugar diet and doing a lot better. I am able to leave the house 2 to 3 times a week and can recover from exertion. For me sugar, dairy and white carbs are absolutely essential for survival.
If our energy problems would stem from our inability to use carbohydrates and potentially even fat as a source of energy, then this leaves us with a problem of numbers.
As in an average non-ketogenic diet about 60% of calories comes from carbs, it would make sense that the remaining 40% is insufficient. But what goes in must get out or accumulate.
If carbs and fat are not excreted at a far higher rate (compared to healthy people) in our excrements and urine then it either gets stored as fat/body tissue or used as a source of energy. I see no other clear option. Poor and only partial combustion is no alternative: if for example pyruvate is not dumped out of our bodies, it must be at a point converted back to glucose (that must be used for energy or stored) or directly used for energy.
As we do not as a group have the tendency to keep gaining lots of weight each month, it must be used as a source of energy. So if we can’t metabolise carbs and fat, is that only during peak load? If so, the following problem remains: we are restricted to loads that are for healthy people far below the point where fat can’t fulfill their basic energy needs. So if our only problem were that we can’t metabolise carbs and fats fast enough for peak loads, we still should be able to keep going at a moderate paste which most of us can’t either.
I have a question for Cort and all the brilliant people here. I found a supplement by Swanson labeled Acetyl-CoEnzyme A precursor complex. It says it contains a proprietary mix including calcium pyruvate, L-cysteine & magnesium malate. It is not expensive.
Does this sound like it would help those of us with CFS-ME?
I have no idea but maybe someone else would…
You may want to check with your doctor before starting supplements but they’re generally safe to try. Dr. Steven Sinatra, a cardiologist who also works with ME/CFS patients researched amino acids and wrote “Metabolic Cardiology” which explains how a number of these amino acids work. it’s available on Amazon. He has two websites: https://heartmdinstitute.com/ and http://www.drsinatra.com/immune-health-2/ He sells supplements, but his recommendations can be followed by buying any brand. Magnesium glycinate is the most bioavailable, but this article suggests malate may have benefits for ME/CFS: https://www.verywell.com/magnesium-malate-fibromyalgia-chronic-fatigue-715798
@ Pam. I feel rather out of my depth here, but if I’ve understood Cort’s review correctly, we have too much pyruvate, and therefore the ‘calcium pyruvate’ wouldn’t be helpful to us, but perhaps the l-cysteine and magnesium malate would (I have found magnesium malate/citrate to be helpful, especially in combination with vitamin B6). But maybe someone with more knowledge could comment.
Cort also mentioned that the amino acids Ile, Leu, Phe, Tyr, Lys and Trp were significantly reduced, but only in women, so if I were a woman, I would probably try supplementing those very gradually and see if there were any benefits (unfortunately I’m a man ;)).
Looking further at the pyruvate dehydrogenase/pyruvate dehydrogenase kinase issue, surely if pyruvate dehydrogenase kinase is overactive, an obvious possible intervention would be a PDK inhibitor – e.g., dichloroacetate? https://en.wikipedia.org/wiki/Dichloroacetic_acid
https://en.wikipedia.org/wiki/Pyruvate_dehydrogenase_kinase
Has anyone tried dichloroacetate? Or has anyone got any other ideas for interventions on the basis of the above research?
Thank you both!
I note that Drs. Fluge and Mella have a patent on their B-cell theory of ME/CFS. Not sure how that would work s regarding any possible future profitor if their profits would be wholly turned over to a university and more research?
Is this pyruvate theory anything like the PYR test at Mayo Clinic, “Screening for possible disorders of mitochondrial metabolism, when used in conjunction with blood lactate collected at the same time to determine the lactate-to-pyruvate ratio”? http://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/8657 Where instead of an inherited structural disorder, the disorder would be considered brought on by autoimmunity?
We’re engaging in an interesting process collectively online, (which I’m quite enjoying). May I add my two cents: at this level of consideration, we must get L-LACTATE, D-LACTATE, and LACTIC ACIDOSIS correct. They are all distinct and the only bad thing in the three of them is lactic acidosis. This is a great tutorial:
http://www.mayoclinicproceedings.org/article/S0025-6196(13)00555-7/fulltext#sec4
“Elevated lactate levels are not clearly and universally defined…The terms lactate and lactic acid are often used interchangeably, but lactate (the component measured in blood) is strictly a weak base, whereas lactic acid is the corresponding acid. Lactic acidosis is often used clinically to describe elevated lactate levels, but it should be reserved for cases in which there is a corresponding acidosis (pH <7.35).12 The exact pathogenesis of elevated lactate levels in various conditions is likely multifactorial, patient specific, and disease specific. In general, lactate level elevation may be caused by increased production, decreased clearance, or a combination of both…"
You know that tired explanation we keep trotting out, that we go into anaerobic cell respiration when our body runs out of oxygen? Not so. There's a new understanding that aerobic and anaerobic cell respiration happen AT THE SAME TIME. This newer theory says that L-lactate is used as a fuel which kicks in when we push our bodies to the max, and lactic acidosis happens when our liver can't clear the acid quickly enough from the lactate fuel burning process. There's nothing wrong sour bodies choosing lactate as a fuel when we're pushing it to the max. Elite athletes do interval training in order to "teach" their bodies to choose lactate which gives them more power in shorter periods of time. (the question for ME/CFS is WHY are our bodies deciding to choose lactate as fuel so soon). https://www.ncbi.nlm.nih.gov/books/NBK22417/
The Me/CFS exercise trainer I saw told me that we might as well learn how to go with our bodies flow if our body is deciding to use lactate as a fuel. So we train like elite atheletes. Which is, we push in intervals. Which is a whole discussion on its own. There's a whole runners community which has become very conversant about how to use more and more L-lactate as fuel while minimizing lactic acidosis. http://www.runnersworld.com/tag/lactic-acid
D-Lactate is produced in the gut from undigested carbohydrates. The reason these carbohydrates go undigested varies from person to person. Before we diagnose ourselves with short bowel syndrome, keep in mind it could be as simple as overeating.
I'm digressing, and I'd like to get back to that Rituxamab study. These Rituxamab studies have left a lot of damaged and dead people in it's wake, whether for ME/CFS or cancer or multiple sclerosis. Since Vitamin D plays a very effective role as a natural Rituxamab, I would think that governments would insist that anyone with an autoimmune condition considering Rituxamab should be tried on a Vitamin D protocol first. http://www.dummies.com/health/nutrition/vitamins/how-vitamin-d-influences-the-bodys-immune-system/ I really don't understand how a therapy which causes cytokine storm deaths are working for anyone with an autoimmune condition. Perhaps someone could enlighten me? And not with "self-reported" endpoints. With real science. I'd also like to know if researchers or the Rituxamab manufacturer are keeping data in order to figure out what they call in media the "mystery" of Rituxamab deaths. It seems to me that cytokine release of patients who experience edema, heart failure, etc. are swept under the rug as "rare" and attributed to "pre-existing conditions". No kidding! https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3364040/ http://www.iwmf.com/sites/default/files/docs/Rituximab-Associated_Neutropenia-Seminars_in_Hematology.pdf
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3002645/
Wow, I just want to thank you for your information. What you have said makes sense to me, clearly.
I take vit D and am a candidate for Rituximab, but will definitely re-think and research first.
I am under as huge amount of stress right now (family death) and want to be as pro-active as possible to keep my ME/FM symptoms under control.
What has helped you? I take Vit D (and others like B vits) but how much? Anything else that might help?
You’re welcome! Glad that you’ll be making your decision equipped with more information. We create more personal power that way. Sorry to hear about the death in your family.
The clinicians/researchers who were at the IACFSME conference have been finding that it’s a number of things that make patients rotate around health rather than sickness. I heard the same story from patients who went from housebound to functioning outside of the house. Me too. Think homeostasis rather than one cure treatment. A lot of people who get better can forget all the little changes that they made that helped get them back to health. Homeostasis is like we’re riding a bicycle which is always in motion. We’re constantly balancing taking in fuel and clearing toxins. If we get out of balance, it’s like we’ve fallen and need to slowly get up again.
I took 10,000 I.U. Vitamin D (liquid AOR brand), 200mcg Vitamin K (AOR brand and that’s micrograms, not milligrams), and 4-7 100mg magnesium glycinate pills (Douglas Laboratories). I took these brands to avoid fillers and additives. I took them for 2 months and had my vitamin D and calcium levels checked as I didn’t want to overdo the vitamin D and I wanted to make sure that hypercalcemia didn’t happen from too much vitamin D. Everything was fine so I continued for a total of 3 or 4 months then I cut back and took amino acids for a few months. The amino acids I took was a mitochondrial cocktail designed by Dr. Steven Sinatra (you can get the supplements anywhere,you don’t have to buy his): CoQ10-300mg, alpha-lipoic acid 300mg bid, acetyl-L-carnatine – 500mg to 3000mg, magnesium glycinate 400-800mg, N-acetyl-cysteine 500mg. Dr. Sinatra recommends a number of different mitochondrial cocktails which can work; this is just one I tried. He also suggests Dribose, but some studies suggest that it’s better to take Dribose precursors instead to let the body make its own Dribose. The latest supplement people are trying is Alpha-GPC which boosts acetylcholine in the brain and the body. It’s used in an effort to help Alzheimers patients and it’s used by bodybuilders. All supplements (also called nootricueticals, amino acids) have diminishing returns. Use them for awhile before a cognitively or physically challenging task, then you’ll feel like you want to put them aside and use them only once in awhile. The body was really meant, for the long term, to make its own amino acids from food.
I changed my diet to a big variety of veggies sprayed with no or as little pesticides as possible and a little antibiotic, hormone free meat allowed to graze on pesticide free grass. I don’t take in carbohydrates laden with sugar like pasta, rice and bread and soft bananas. I eat bananas just ripe (and not a lot) and some veggies and nuts are high in carbs without the added sugar.
I decreased my toxic load from industrial chemicals as much as possible. I use Ecover brand dishwashing and laundry liquid and floor soap, and otherwise castille soap, vinager and baking powder cleans everything. I have a great recipe for natural deodorant.
I sent away for the 23andme.com kit and when I got back the results from my saliva there was some good info right away that let me know I’m caffeine sensitive. I had already cut out coffee but it was good to know I was on the right track with that. There’s also a section in the results which shows which medications an individual may be more sensitive to or less sensitive to.
I take an aquafit class 2 or 3 times a week if I can. I’ve told the instructors I have ME/CFS and that I need to rest every once in awhile during the class – I just hang out and float for a few minutes when I feel my heart rate up. When I do my own routine I stretch gently, (Dr. Peter Rowe has found overstretching limbs can cause a ME/CFS flare) and then do 1 minute intense exercise for 8 reps then stretch gently again. The water suspension helps prevent too much histamine and lactic acid production and the exercise gets the acetylcholine we’ve taken in through food and/or supplements through the body. This trains our body to have more energy. It’s a slow but fantastic process.
I’ve been pursuing looking up the link between low acetylcholine levels at birth (for various reasons) and whether Ehlers Danlos Syndrome is related. EDS-and there’s various “types” -is the result of not having enough collagen in the body and depending on which body system is affected, a person is considered in one “sub-group” or another. I’ve been looking up links between types of collagen and acetylcholine. A number of EDS patients also have POTS and other ME/CFS symptoms. Rituxamab targets one type of acetylcholine receptors. It’s also been tried on myasenthia gravis patients, who are born without enough acetylcholine receptors. There’s something rituxamab manufacturers know about acetylcholine! I’d just rather eat foods that will help me produce my own acetylcholine. Raw eggs have a whopping high amount. I get eggs from a local farmer I trust and I wash them in mild dish soap and warm water before using them and sometimes dunk them in boiling water to kill any pathogens on the shell. Here’s an article about foods with high acetylcholine: http://www.livestrong.com/article/392875-food-sources-of-acetylcholine/
It’s a process to health! And many companies hoping to profit off our disease. To each their own health choices, as long as we have all the information! Looking forward to being in touch with you again on healthrising
Replying to LY
Thank you so much for all the information you’ve shared.
I do take some of the mentioned supps but there are a couple that I don’t have so will be starting them.
I used to swim a lot and miss it greatly but I don’t even last 5 minutes then PEM sets in and I literally have gotten lost driving home!
I haven’t tried in a couple of years so I will restart really slowly.
Thanks again for your good sound advice.
I can’t always swim, either Katie! I’m upright in the pool. I walk, I run, stretch gently; I get my heart rate up slowly then faster if I’m able. It’s a fine line. Getting our heart rate up helps our metabolism (which circulates whatever we’re taking into our bodies, good and bad) but too much and we’ll feel sicker. Over the arm swim strokes and kicking from the hip horizonally is probably too intense for you right now. Maybe start out with the help of a noodle or flotation belt and stay vertical.
Definitely check with your doctor and maybe a pharmacist before starting supplements!
We can have our genes checked at 23andme.com ($250) and let genetic genie (donation basis) run through them for some information about our genetic predisposition. Not sure if it’s 100% accurate, but told me what I know about myself- caffeine sensitive, glutathione is fine, some trouble with acetylation.
Hi again,
I’ve had my genes done by 23 and me (and translated)-interesting results.
I wasn’t swimming-just walking and stretching.
Unfortunately there is not a salt water pool close by. It’s 130 km away.
Have you heard of Watsu therapy? I used to do that in Vancouver but now live quite far away from there. I’m searching for another place.
Yes, I had aqua massage once. Almost fell asleep. Though I can sleep anywhere!
Not sure how close you are to Victoria:http://www.pilatesvictoriabc.ca/aquatherapyhydrotherapy/
Sometimes hotels or Inns will have a day rate or monthly fitness membership that allows access to their pool.
Maybe this to start with milky epsom salt bath after (except for the stretch bands): http://www.sitandbefit.org/
Found this online, thought it was interesting: http://fibromyalgiaflotationproject.com/
http://floathq.com/
Not exercise, but seems helpful for FM pain.
I don’t trust the Mayo Clinic description. It looks distorted to me.
They are right in that aerobic and anaerobic metabolism occur at the same time. On the other hand this has never been disputed in the science, and is not new. What is important is that there is a sudden spike in lactic acid production at the point where oxygen utilization becomes inadequate.
Lactic acid is NOT fuel. It has no appreciable value as fuel. The PRODUCTION of lactic acid involves conversion of NADH to NAD+, and this is the primary factor as to why its important. Without sufficient NAD+ energy production will slow. Lactic acid is mostly reconverted to glucose at a considerable energy cost. Its anti-fuel.
Its worth noting that concentrations will not rise until the rate of clearance or conversion is lower than the rate of production.
A secondary factor is changes in oxygen dissociation, when acidity rises enough to cause increased oxygen dumping. However if prolonged it can cause a change in the enzymatic production (and hence 2,3 BPG or DPG) and this causes lowered oxygen release. Lowered oxygen leads to more lactic acid which leads to even worse oxygen release, triggering lactic acidosis. The typical time frame is three days.
Acidity is very low for lactic acid and its not clear that it will normally trigger acid sensors in exercise. Indeed, carbonic acid is more likely to be the issue, due to poor carbon dioxide clearance.
Lactic acidosis occurs when there is a long term shift in the capacity to clear lactic acid. Typically this occurs when increased acidity over time affects the production of 2,3 DPG. Other things can do this though, like poisons or mechanical damage to circulation, primarily by lowering oxygen supply.
To date nobody has died of Rituximab in treating ME, at least to my knowledge. Rituximab deaths, as a percentage of those receiving only Rituximab, appear to be very very low. Most cases of death are for patients on cocktails of multiple drugs. When combined with other drugs the risk appears to increase. However if improperly administered then its very dangerous, but this is an acute reaction and sometimes results in the drug infusion being stopped, maybe in about 10% of cases. This is why its infused slowly over hours. Its not an injection.
Why don’t they use vitamin D to treat lymphoma? I doubt its effective at eliminating B cells. There is however a proposed statistical association between lymphoma risk and lack of sunlight. This is a risk of developing lymphoma from low vitamin D, presuming its correct, and not about treating it.
One of the factors being examined in the phase 3 Rituximab trial, for which results will be unblinded in about October this year, is safety. Rituximab is probably not advisable outside of a clinical trial at this point, including off-label use. This is a discussion that can be had some time next year (2018) after publication of the results.
There is always uncertainty in medical interventions. That is one of the reasons we have RCTs. Those are what will give us the data we need. Are there any successful RCTs of vitamin D therapy for these kinds of uses? I know its been needed, but I do not recall reading one that definitively supports the use of vitamin D even as an adjunct therapy. That is not to say this proves it does not help, that is a different question.
I live in a part of the world where it is known that drinking too much water can be lethal. Its the conditions under which this occur that are important. Rituximab might yet be shown to be too dangerous for use, but its not clear that it is as yet.
In treating ME and CFS I would be more concerned about cyclophoshamide. Fluge and Mella report the side effects seem to be more severe than they see in cancer patients.
Alex, no one has said, not in my comment and not in the Mayo Clinic description of lactate, that lactic acid is fuel. Lactate is fuel. Higher levels of lactate can be a sign of lactic acidosis but not necessarily if the body is able to use the lactate as fuel efficiently. Please read again carefully. Even Fluge and Mella do not mix up lactate with lactic acid.
Oops, the arrows all vanished in that buffering equation. Its readily found online. So let me try again:
H+ + HCO3 H2CO3 H20 + CO2
Please note this entire equation is in water solution.
I hope this is not a repeat post, I already posted a correction but it did not show. The buffer equation did not copy right, it should be:
H+ + HCO3 H2CO3 H20 + CO2
I am sorry but that article is about science that is quite old now, and I agree with, and supports my view.
What I want to know about is the NAD-NADH-Sirtuin 4- Lactic/lactate-pyruvate chemistry. I do not think its a coincidence that Sirtuin 4 is involved. I just found an article on the NAD-Sirtuin link that I have to look into, involving mitochondrial calcium, but interpreting the energy issues cannot happen without taking the broad mitochondrial data into account, as well as the inhibiting chemistry.
Let me say again, these theories are old, it just took a long time for sports writers to get a handle on it.
Are you familiar with the lactate shuttle hypothesis? Lactate can be oxidized to pyruvate by LDH in the mitochondria. According to wikipedia, this process accounts for the majority of lactate turnover in the human body (versus the Cori Cycle), and it is proposed that 75-80% of lactate is recycled through this process during strenuous exercise.
Here’s a recent study that seems to confirm it.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5069139/
Great to hear all this news after 30 years of being told it’s all in your head. I had gamma globulin infusions in the late 1980’s with no improvement but the one thing I know is that a high carb diet weakens me intensely – forget bread! I crave red meat, especially lamb. Also, all the fat on it! And salt, yes, I have to have lots of it. The searing pain across the back during a short walk is something I had put down to aging in the spine – interesting.
In human physiology lactate is just the (conjugated base) buffered state of lactic acid. There is a limit to what can be buffered, which involves hydrogen (acid) and when this is exceeded lactic acid rises. This is part of the mechanism of lactic acidosis. While buffered as lactate the lactic acid is not a problem. When lactic acid clearance is high this is also not a problem. When the buffer capacity is exceeded then you can get acidity if clearance also does not rise or production does not fall.
Normally what happens is that lactic acid is buffered to lactate and transported via the blood. Its when this capacity is exceeded, whether by excess lactic acid production, or reduced clearance via the blood, that acidity can rise. Such acidity is probably not an issue in the short term, and is helpful in itself as I already explained.
It was your comment LY that cited lactate as fuel, not the Mayo Clinic article: “(the question for ME/CFS is WHY are our bodies deciding to choose lactate as fuel so soon).” My arguments apply equally to lactate. It can be converted to fuel but at some energy cost, such as in the Cori cycle. Its overall draining the body of energy. Its the NAD+ that is produced in lactic acid production from pyruvate that is important.
Many textbooks cite production of pyruvate to lactate, but this obscures the actual chemistry. In proper physiological buffering most lactic acid should be as lactate, but you cannot interpret this outside of consideration of buffering. If the buffering is overtaxed then more will be as lactic acid. If this were not the case then lactic acidosis would not exist. It exists because buffering limits are exceeded, though why they are exceeded can be from a range of over-production and under-clearance issues, or a failure in buffering.
So far as I am currently aware its the bicarbonate and carbonic acid buffer that is doing most of the work. H+ + HCO3 H2CO3 H20 + CO2 Breathing out CO2 decreases acidity by shifting the buffer balance. I could be wrong about this though as I have not investigated this stuff in any depth for a decade and a half now. There might be other mechanisms at work.
High lactate can occur in the absence of lactic acidosis. The acidosis only occurs when buffering, production or clearance have failed, and a failure in buffering is always present.
Lactate is not fuel unless you consider the Cori cycle and its high energetic cost, and that is a very indirect path when considering a cell.
The software on this site removes the arrows in my equations even though they are in ascii. I don’t know why. The bicarbonate buffering equation is easily found in many places. I also posted the correction next to the wrong post, my bad.
Alex, I suggest you investigate d-lactate, l-lactate and lactic acid latest research. The theories have really changed lately and we have sports medicine and science to thank for this.
Here’s an article to sink you teeth into to start which will help you with an overview:
http://www.sport-fitness-advisor.com/lactic-acid.html
The reason I press the point here is that understanding this is really the key to understanding how we can push ourselves in intervals to get out of bed, get out of the house and participate in low histamine activity. By training our bodies to use more lactate as fuel with decreasing lactic acid buildup. It’s not the whole answer for ME/CFS, but it’s an important stage to get through as we get back to homeostasis.
See my comment on Sirtuin etc. that is posted in the wrong place again.
Indeed, there’s value in considering lactate as fuel. Here’s a quote from the above article I linkedhe numbers are footnotes you can reference on the site:
“The heart, brain and most slow twitch fibres are very apt at clearing lactate from the blood to the extent that they prefer lactate as a source of fuel (27,28,29). Note however, that lactate must first be converted into pyruvate before it can be used as a source of energy.”
If you read the latest literature and still think it’s not valuable for an individual ME/CFS patient to choose to maximize their ability to increase their capacity to use lactate as a fuel simply by pushing then resting alternately, then I understand why you come to your conclusion, and it seems you prefer that we wait for a substance to increase our energy? I can tell you from my experience, increasing my anaerobic capacity along with other lifestyle and diet changes helps me function better while waiting for that elusive cure.
BTW, are you a fellow patient? What’s helped you?
Lactate is extremely poor if you consider it as fuel. Its not a major issue. Its a false path in my view. The body does have many paths for lactate clearance, but the main one is the liver, followed by kidneys. The requirement to convert it to Pyruvate is the telling issue here, its a high energy process. This can be very important with respect to NAD/NADH however.
I have moderate ME and until two months ago was heading for severe and worse. I am also a biochemist who has been debating these issues with scientists for maybe seventeen years now.
Pacing is the only thing that has helped me consistently, though many treatments help for a short while. Most tend to be antioxidants or pre-antioxidants, though they often have other functions.
Exercise training in ME is extremely problematic. Aerobic training fails, period. Most of the claims that exercise helps are debunked, and some may be retracted. Now I do not doubt that some exercise, done right, may help. The exercise physiologists are still trying to figure that out. If it induces PEM then it is probably a bad idea.
In particular any reference to GET as used by (BPS/GET/CBT) psychs is severely discredited. Its psychobabble applied to exercise physiology.
There is no credible evidence that improved lactate clearance will improve ME much, and definitely none for a cure. Its not a bad thing to have,and is associated with increased longevity, but very hard for anyone with ME to achieve. Many with idiopathic chronic fatigue will probably benefit however. ICF and ME are not the same.
Indeed Workwell reported a case study of a female athlete who steadily increased her training regime over a year. At the end she was less fit than at the start. She finally followed their advice, and this excludes aerobic training.
Thanks for sharing. I’m glad your ME that was worsening took a turn for the better. Seventeen years of debating – you must be somewhat frustrated by now at the lack of real progress. It’s a shame that the sciences are so competitive. There’s something that the Rituxamab researchers understand about acetylcholine receptors and a number of related conditions they’re doing trials on – MS, Myasenthia Gravis, and ME. They definitely understand Vitamin D’s effect on T and B cells, and I think it’s reprehensible to not ensure that a patient’s tried a Vitamin D therapy before trying Rituxamab on them. I’m sure you don’t need to read this article but I’ll post it for anyone else who would like to read: https://blogs.scientificamerican.com/observations/another-reason-vitamin-d-is-important-it-gets-t-cells-going/
I came across a study yesterday which is the most interesting one so far to me on phenolic acids. Alzheimers patients also have acetylcholine issues even before they display symptoms – the theory is that a lifetime of anticholinergic drugs may trigger Alzheimers. It seems to me the treatment would be a preventative rather than a cure. https://www.ncbi.nlm.nih.gov/pubmed/23184188 I’ve been wondering if there’s a genetic predisposition involving acetylcholine in ME,MS,EDS,Myasthenthia Gravis, Alzheimers and ASIA. Which leads to an autoimmune condition upon certain environmental exposures.
Regarding your exercise comment – I’m definitely not talking about GET! I’m talking about making the best of a bad situation. Pacing yourself is accepting that your body is choosing lactate as fuel too often and working with it. It’s a slow and maddening process while we wait for a cure. Working at the gym is indeed too harmful – I tried going slow but longer on the treadmill and I ripped a muscle in my foot. (Probably EDS related.) Yes, if we have ME the histamines get raised too high and we can’t get the elevated heart rate without paying too dearly. I’ve traded the gym for a heated saltwater pool and exercising in intervals. Can’t always do it though; as other health problems come along or chemical exposures from other people’s perfumes, etc, I have to lie in bed until my body detoxes or take it easy while I take an herbal tincture. It’s extremely tough to get back up and out again! And it takes time out of the day and requires body awareness. But even for someone who’s bedbound there’s a wealth of O.T. knowledge built up in the geriatric world. It just seems to me that so many are popping medicines like candy as a first-line treatment in a quest for energy until a cure is found.
Alex, I forgot to mention – I needed to take amino acids at first to be able to do even a short pool interval workout. Don’t need them anymore. I’m too weak to walk upon exiting the pool so I sit at the side for a couple of minutes. I take magnesium afterwards to minimize lactic acid and pain.
I have two questions for you LV.
First, where did you find a saltwater pool? That seems like an impossible dream!
Second, is it possible to translate the information you and Alex have been discussing? I am so far behind, I don’t know where to start.
Pam,
In a nutshell: I’ve been talking this: http://www.runnersworld.com/workouts/workouts-to-improve-lactate-clearing-rates
Not graded exercise training. Not even going to the gym. Just pushing a little beyond what we can do physically and in the process our body will produce less lactic acid. Even at nursing homes, bedbound patients are made to move a little each day by an occupational therapist as part of physical rehabilitation.
Pam,
I’m in Canada – some of the fitness centres have converted from chlorine to saltwater pools. I could try to help you find one in your area online if you let me know where you are.
Cort, maybe you could explain to Pam what Alex and I have been debating? A neutral party! I loved having this debate, BTW. This is how we challenge our assumptions and get to better answers.
Rituximab Study is of major interest. I’m the only PWC who’s had ~50 (Fifty) Treatments…. there have been some mitigations, but who knows the long term effects? Also this year of Treatment necessitated massive Steroids – both oral & IV – so I gained over 150 pounds, not a good side-effect. But am hopeful that they’ll be able to figure out HOW Rituximab works to discern more effective Treatments!
Suellen T, have you given your story on healthrising? I’d very much like to read it and others who have had Rituxamab treatment. Especially how you got CFS, other meds, your diet.
Can a Person just take Acetyl Co A Tablets?
Also what is the best way to deal with Lactic Acid Build Up?
Can we just take Sod. Bicarbonate tabs?
Gerry,
It’s complex. Supplements work, but they have diminishing returns. Our body seems to prefer to synthesize its own products for the most part. Trying out a diet high in organic food high in phenolic acids for a couple of weeks and seeing how you feel is low risk. A word of caution though: Don’t think more is better. Eating too much antioxidant foods is also unhealthy. http://foodwatch.com.au/blog/super-foods/item/top-100-polyphenols-what-are-they-and-why-are-they-important.html Besides these foods, about 10% organic meats will provide the carnatine for the electron transport chain to move nutrients in order to create energy.
Lactic acid buildup – I warm down, stop and rest, hydrate and take magnesium glycinate.
None of this is an answer, though. It’s just making the best of things until truly groundbreaking research is done.
Regarding the citric acid cycle and glucose, sleep deprivation can have a massive impact on these and metabolism/hormones. Most people with ME/fibro experience low or poor quality sleep. Could these problems all arise due to the sleep problem which, again, is a symptom and effect, but not getting to the core of problem. I worry that these are side issues caused by the illnesse’s impact on sleep
Interesting thought, Suzie. My struggles experiencing non-replenishing sleep preceded my huge energy collapse by years.
Hello all – I’m not sure whether anyone is still following this thread, but if anyone who understands the biochemistry is listening, could you help me with whether there’s any difference between metabolising sugars to make energy and metabolising them to make body fat, please?
Reading the post made me resolve to cut out added sugar from my diet, as I was always a bit over-fond of it and this research seems to show that it’s not even useful to me. I was also about 8lb over the healthy weight for my age & height, and I hoped it might halve that! What happened in reality was that, without making any other changes to my diet or routine, I’ve lost 23lb over the last four months.
It doesn’t entirely make sense to me, though. If my body can use this sugar to make fat, why can’t it use it to make energy? Perhaps it’s a completely different metabolic process, I don’t know…thanks for any clarification you can give!
I don’t know but if that helped you might try a ketogenic diet. Some people do find that a ketogenic diet really helps. You can find some resources on it here – https://www.healthrising.org/forums/threads/ketogenic-diet-resources.2176/
Thank you, Cort – I hadn’t realised you’d replied, as I think the notification might have ended up in the spam folder. I’ll certainly take a look at those resources. All good wishes. -Kitty
Women have also problems with Acethyl-CoA production via fat (b- oxidation)… but when I’m a man with CFS – would high fat be a cure???
Interesting thought, Steve – I didn’t know about the Acetyl-CoA thing (I don’t understand much of this stuff!)
I have been suffering from CFS for the last 14 years, I have gradually deteriorated. the initial episode was viral infection with reactive arthritis that all settled but energy level never came back. one thing that I have noticed is that in the initial stage I could work all day with half the tab. of paracetamol, then after a while one tab, with a further interval I had to take two, at last two three tomes a day eventually bringing me to the stage that I could not work. even now after such a long time overexerting leads to malaise, lethargy, increase in body temperature,so called post exertion symptoms are relieved to some extent with paracetamol. now all this will help me to think that is there a cytokine being produced either regularly or intermittently because malaise and lethargy are caused by cytokines. In acute viral infections we find acute lack of energy, may be a lot is used by immune system but largely it is the result of oxydative stress. we also find that if one exert more in acute infection one takes longer to recover much like CFS patients but in a different way. I strongly believe that the mitochondrial dysfunction in CFS is secondary to some thing circulation in the blood and we need to find that circulating devil causing havoc in the body
also could some one advise on the benefits of ketogenic diet, I believe it will not work as two large organs like muscles and brain need glucose readily available to function
Have there been any follow ups to this, or treatments that came of it? Ran across this article 7 years later and I’m curious.