

This is the third of a series of posts on non-thyroidal illness syndrome, NTIS – a pattern of altered thyroid hormone activity often present in severe trauma and critical illness. A recent study suggests NTIS may be present in chronic fatigue syndrome (ME/CFS) as well (Ruiz-Núñez et al., 2018). Dominic provides more evidence for a potential link between the two syndromes when he shows that two factors which studies suggest play a role in ME/CFS (oxidative stress and cytokines), appear to be responsible for maintaining prolonged or chronic NTIS as well. The treatments trialed in ME/CFS and prolonged NTIS are also similar.
Note! This is a long and technical post – you may want to print it out.
Summary
Researchers describe a “vicious circle” involving cytokines, oxidative stress and reduced thyroid hormone activity to explain chronic “non-thyroidal illness syndrome” (NTIS) – a condition that can occur in response to virtually any severe infection, trauma, illness or surgical stress.
Similar patterns found in ME/CFS suggest that the five decades of NTIS research may be able to inform our understanding of the mechanisms underlying ME/CFS as well.
Furthermore, the treatments trialed for NTIS are also similar to those suggested by some ME/CFS practitioners. They include supplementation with thyroid hormones, administration of hypothalamic releasing factors, supplementation with anti-oxidants, and modulation of the immune system.
Introduction
When I was researching what could improve my wife’s debilitating condition, I found that most ME/CFS practitioners treat the immune system, some address the action of thyroid hormones, and a few practitioners target both the immune system and thyroid hormones (see my previous blog post). Dr Sarah Myhill, for example, believes immunological issues largely underlie ME/CFS, but also describes the complete recovery of patients using thyroid hormone supplementation (Myhill, 2018).

NTIS is a pattern of altered thyroid hormone activity that can occur in response to virtually any severe infection, trauma, illness or surgical stress
This made me interested in understanding the relationship between the immune system and the action of thyroid hormones at the cellular level. I was particularly interested in learning if the relationship between the immune system and thyroid hormones could produce a feedback loop that leads to a patient becoming “stuck” in an ME/CFS-like illness state.
I found that the relationship between inflammation and thyroid hormone activity has been studied for decades and continues to be studied in the context of a condition called “non-thyroidal illness syndrome” (NTIS) – also called “euthyroid sick syndrome” or “low T3 syndrome” – often present in severe trauma, critical illness and following surgeries. Moreover, I learnt that some of the more recent NTIS research describes “vicious circles” mediated by cytokines, oxidative stress and thyroid hormone activity that keep some patients ill in a form of “prolonged” or “chronic” NTIS (Mancini et al., 2016; Chatzitomaris et al., 2017).
Furthermore, to my surprise, there are many physiological similarities between ME/CFS and NTIS, including: down-regulation of metabolism, reduced mitochondrial activity, oxidative stress, distinct cytokine signatures, altered immune cell activity, dysregulation of adrenal hormones, etc. (see Table 1 at the end of the blog post).
In this post, I will argue that the findings stemming from five decades of NTIS research will likely provide important insights into the mechanisms underlying ME/CFS.
First, I provide an overview of some of the mechanisms described in the literature on NTIS, specifically the “vicious circle” described by some researchers (Section 1). Secondly, I summarize some of the main implications of increased cytokines and oxidative stress level, and depressed thyroid hormone activity — and similarities with ME/CFS (Section 2). Finally, in view of their potential application for ME/CFS, I briefly present the ongoing debates regarding NTIS treatment (Section 3).
Although this blog post is focused on the mechanisms related to alterations in thyroid hormone activity, it’s important to note that other endocrine systems are also altered in trauma and critical illness, specifically the growth hormone and adrenocorticol axes (van den Berghe, 2016). Research in the field of critical care medicine on these endocrine systems and other aspects of critical illness (see Annex) may also be relevant for understanding the mechanisms of ME/CFS but are beyond the scope of this blog post.
Section 1: Mechanisms in NTIS
Starting in the early 1970s, clinicians working in intensive care units observed that patients with a wide range of critical conditions – starvation, severe burns, head injuries, sepsis, heart surgery, renal failure, etc. – had low plasma concentrations of the active form of thyroid hormones (T3), and high plasma concentrations of inactivated thyroid hormones (reverse T3; rT3) (Warner and Beckett. 2010).
Over time, it became clear that this pattern of altered thyroid hormone concentrations can occur in response to virtually any severe infection, trauma, illness or surgical stress (De Groot, 1999; Wajner et al., 2012). Clinicians gave this condition the name “non-thyroidal illness syndrome” (NTIS), also called “euthyroid sick syndrome” or “low T3 syndrome.”
Recognizing that thyroid hormones regulate the rate of our metabolism, NTIS was initially described as a state of “protective” down-regulation of metabolism during times of duress – i.e. a form of “hypometabolism” to save energy (Carter et al. 1974).
However, the initial assumption that NTIS is a “protective” down-regulation of metabolism is increasingly being questioned by NTIS researchers – particularly in the case of chronic or prolonged rather than acute or transitory cases of NTIS (Boelen, 2011; van den Berghe, 2016).
As the mechanisms involved in NTIS were elucidated, researchers increasingly began to suggest that chronic NTIS was a “maladaptive process.” Irrespective of the initial illness or trigger, some patients appeared to get stuck in a chronic, hypometabolic state they had difficulty escaping from (Plikat et al., 2007).
Based on nearly five decades of NTIS and related studies, researchers have proposed a model that describes how the vicious circle which keeps some people stuck in a prolonged or chronic state of NTIS occurs. This model suggests that this hypometabolic state is maintained by reciprocal relationships between inflammatory cytokines, reduced thyroid hormone activity, and oxidative stress (Mancini et al., 2016; Chatzitomaris et al., 2017).
Figure 1: Simplified model of NTIS, based on the paper by Mancini, et al. 2016 (see the paper for the more complex model).
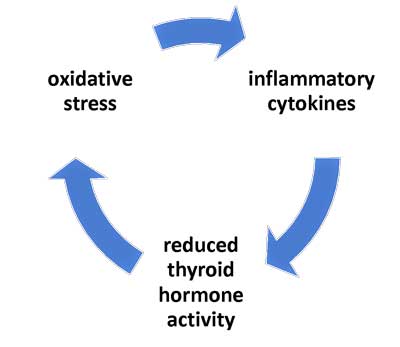
Figure 1: First thought to be protective mechanism brought on by severe illness, prolonged NTIS is now thought to be a maladaptive response occurring when cytokines and oxidative stress impact thyroid hormone activity
This simple model doesn’t address all aspects of NTIS, nor does it incorporate the associated changes in other endocrine systems during critical illness (such as the adrenal hormone system), but it does serve as a good introduction to the bulk of the literature on NTIS.
The key elements of this suggested “vicious circle” in prolonged NTIS include the following mechanisms:
- cytokines depress thyroid hormone activity;
- low thyroid hormone activity contributes to oxidative stress; and
- oxidative stress stimulates the production of pro-inflammatory cytokines – thereby completing the circle.
I will explain these mechanisms in detail in the next three sub-sections.
Mechanism #1: Cytokines depress thyroid hormone activity
Cytokines: Cytokines are low-molecular-weight proteins that regulate the nature, intensity and duration of the immune response and play important roles in autoimmune and inflammatory diseases. Very versatile, cytokines can act in an autocrine (affect the same cell), paracrine (affect cells close to them) or endocrine (affect cells far away) fashion and can affect different cells in different ways. In what’s called a cascade effect, cytokines often stimulate the production of other cytokines. Over 50 cytokines and chemokines exist.
In trying to understand the mechanisms behind NTIS, researchers followed up on a major clue: the fact that alterations in activated (T3) and inactivated (rT3) thyroid hormone concentrations in plasma are associated with alterations in cytokine levels as well (Boelen et al., 1993; Davies et al., 1996; Maura Neto et al., 2016).
In the past few decades researchers have increasingly clarified the ways in which cytokines depress thyroid hormone activity. These can be categorized into sub-mechanisms at (i) “central level” (i.e. “above the neck”) and (ii) “peripheral level” (i.e. “below the neck.”).
I will give a brief overview of these sub-mechanisms in the paragraphs below. However, two key take-aways from this process are:
First, these sub-mechanisms result in tissue-specific down-regulation of metabolism. Interestingly, the metabolism of different tissues (the liver, kidney, brain, heart, adipose tissue, and other tissues) is down-regulated at different rates, perhaps in order of the importance of the organs to survival.
Second, by just looking at the concentrations of activated and inactivated thyroid hormone in the plasma (as is usually the case in clinical settings), we only see the “tip of the iceberg” of the alterations in thyroid hormone activity occurring at the tissue level (Ruiz – Nunez et al., 2018; Donzelli et al., 2016). In fact, the tissue-level alterations in thyroid hormone are often easily missed all together (Dietrich et al., 2016).
(a) “Central level”: how cytokines depress the overall production of thyroid hormones
In chronic NTIS, cytokines targeting the hypothalamus (located in the middle of the brain), the pituitary (located at the front of the brain) and the thyroid glands (located in the neck) can lead to a reduction in the overall production of thyroid hormones.
Under normal conditions, a thyroid hormone feedback system works like a thermostat to maintain stable plasma thyroid hormone concentrations according to a daily rhythm (Fisher, 1996). In brief, when activated thyroid hormone concentrations in the plasma dip below a certain threshold, the hypothalamus produces thyrotropin-releasing hormones (TRH) in order to signal the pituitary to produce thyroid stimulating hormone (TSH), which in turn signals the thyroid gland to produce more thyroid hormone (TH).
Figure 2: The cascade for production of thyroid hormone (TH)
However, in the case of chronic NTIS, cytokines (e.g. IL-12 and IL-18), in association with other signaling factors (including leptin, glucocorticoids, etc.), inhibit the production of TRH by the hypothalamus (Boelen et al. 2004; Chatzitomaris et al., 2017).
It’s believed they do this by “up-regulating” the deiodinase enzymes D1 and D2 which convert the mostly inactive form of thyroid hormone (T4) into the active form (T3) in the hypothalamus. The increased local T3 levels then give the hypothalamus the “impression” that active hormone levels are fine (Joseph-Bravo et al., 2015). It is as if the air around the thermostat was being heated, making it appear the house is warm enough.
As a result, in chronic NTIS plasma thyroid hormone levels have to drop a lot more than usual in order for the hypothalamus to initiate the sequence that (via TRH and TSH) results in the production of more thyroid hormone by the thyroid gland. Researchers call this an alteration in the “set point” of the feedback mechanism between the plasma concentration of T3 and the release of TRH (Chatzitomaris et al., 2017). (Note: this phenomenon also explains why TSH levels can appear normal in blood tests even when plasma thyroid hormone levels are low.)
Moreover, without going into details, in a process called “TSH suppression”, cytokines (e.g. IL-1b and TNF-α) also decrease the release of TSH by the pituitary (Harel et al., 1995; Wassen et al. 1996). Finally, by reducing iodine uptake and thyroid hormone excretion, cytokines (e.g. IL-1) also impact the activity of the thyroid gland itself (Bartelena, 1998; De Groot 1999).
The consequence of these “central” mechanisms – i.e. the alteration of the “set-point” for TRH production, the “TSH suppression”, and/or the inhibition of the thyroid gland – is a general depression in plasma thyroid hormones leading to generalized hypo-metabolism.
It should be noted that these “central” mechanisms come into play during prolonged or chronic NTIS. During acute and early stages of NTIS, “peripheral” mechanisms serve to down-regulate metabolism quickly to help conserve energy resources (Wajner et al. 2012; van den Berghe, 2014). I will explain these mechanisms next.
(b) “Peripheral level”: how cytokines depress thyroid hormone activity in tissue-specific ways
In acute and early stages of NTIS, mechanisms involving cytokines lead to the quick depression of thyroid hormone activity in tissue-specific ways.
Under normal conditions, once thyroid hormones are released by the thyroid gland into the plasma, further steps need to be completed before they can impact the metabolism of the target tissue. Simply put:
- thyroid hormones are first “bound” to thyroid hormone binders and carried around the body;
- cellular transporters then “transport” the thyroid hormones into the cells;
- inside the cells, deiodinase enzymes “convert” thyroid hormones into the active or inactive forms;
- when the active thyroid hormone is then “received” by nuclear receptors, the target cells initiate gene transcription. (Alternatively, gene transcription is halted if an inactivated form of thyroid hormone (rT3) lodges onto the nuclear receptors).
(Note: thyroid hormones can also interact with other elements in the cells to initiate “non-genomic” effects).
An alteration in any of these steps can lead to large “time and tissue-specific” adjustments in cellular metabolism – even without any, or only minor, changes in the plasma concentrations of thyroid hormones (Gereben et al., 2008; Mendoza et al., 2017; Cicatiello et al., 2018).
Figure 3: The path of the thyroid hormones to target tissues
NTIS researchers have shown that cytokines (notably IL-1β, IL-6, TNF-⍺) can impact each of these steps (see review by Warner and Beckett, 2010; Wajner et al., 2012).
Alterations induced by cytokines on the path of the thyroid hormones include:
- changes in the amount and affinity of thyroid hormone binders in the blood (Bartelena et al., 1992; Bartelena et al., 1998; Afandi et al., 2000);
- modifications in the expression of the transporters that bring the thyroid hormone into the cells (Mebis et al., 2009);
- the down– and up-regulation of deiodinase enzymes that convert the thyroid hormone into active and inactive forms, respectively (Bartalena et al., 1998; Huang et al., 2005);
- the variation in the quantity and type (i.e. “isoforms”) of cellular thyroid hormone receptors present (Kwakkel et al., 2007; Rodriguez-Perez et al., 2008; Lado-Abeal et al., 2010).
The relative sequence and importance of these various “peripheral” mechanisms in depressing thyroid hormone activity in different phases of NTIS and different tissues are the subject of most NTIS publications (see the reviews of Warner and Beckett, 2010; Wajner et al., 2012; and Chatzitomaris et al., 2017). (Note: additional mechanisms have also been proposed; see Annex).
Of the above mechanisms, the ones that have received the most attention are the effect cytokines have on the down-regulation of the deiodinase enzymes that convert thyroid hormones into the active form T3 and the up-regulation of the enzymes that convert thyroid hormones into the inactivated form “reverse T3” (rT3).
Notwithstanding the differences found between the tissues, and between acute and prolonged NTIS (Mebis et al., 2007; Boelen et al., 2017; Fontes et al., 2017), alterations in the activity of the deiodinase enzymes during NTIS generally lead to a decrease in the active form of thyroid hormone (T3) and an increase in the inactivated form (rT3) in peripheral tissues. This explains the alternative name for the syndrome: “low T3 syndrome.”
In sum: the various “central” and “peripheral” level sub-mechanisms induced or maintained by cytokines during prolonged NTIS result in a general and tissue-specific reduction of thyroid hormone activity – in other words: hypo-metabolism. Moreover, the observed changes in the plasma concentrations of thyroid hormones are just a fraction of the mostly invisible changes in thyroid hormone activity experienced at the level of the tissues (i.e. are “the tip of the iceberg”).
Mechanism #2: Depressed thyroid hormone activity contributes to oxidative stress
Oxidative stress is the imbalance between the production of pro-oxidant substances and anti-oxidant defenses. In other words, oxidative stress occurs when there are not enough anti-oxidants to neutralize the pro-oxidants in the body. The most important pro-oxidants are the reactive oxygen species (ROS) and reactive nitrogen species (RNS). ROS and RNS are formed as natural byproducts of the normal metabolism of oxygen (c.f. mitochondrial respiratory chain) and enzyme activity, respectively. Enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx), as well as transition-metal binding proteins such as transferrin, ferritin, and ceruloplasmin, prevent the production of pro-oxidants or rapidly inactivate them. Sources: Mancini et al., 2016, and Wikipedia
As described above, researchers have established that through a number of mechanisms, thyroid hormone activity is depressed during NTIS via the mediation of cytokines. This brings us to the second key element of the “vicious circle” in chronic NTIS: low thyroid hormone activity contributes to oxidative (and nitrosative) stress.
Both hypothyroidism and hyperthyroidism have long been associated with oxidative stress (Canon-Europa et al., 2012). In the case of hypothyroidism, the principal mechanism is the inability of the cells to make sufficient anti-oxidants to maintain a healthy oxidative balance. (In the case of hyperthyroidism, the mechanism is the generation of too many pro-oxidants.)
The same mechanism existing with hypothyroidism is at work in the case of NTIS. Specifically, the depressed thyroid hormone activity results in reduced function of two proteins (“Uncoupling Proteins-2 and -3”) with anti-oxidant properties (Bianco et al., 1988). In addition, low cellular thyroid hormone levels alter the lipid concentration of the cell membranes which in normal conditions maintain the cells’ oxidative balance. Finally, as a result of low thyroid hormone activity the mitochondria which have been damaged by oxidative stress are not cleared out (cited in Mancini et al., 2016). In sum, during times of low thyroid hormone activity, intra-cellular oxidative stress increases.
In turn, oxidative stress inhibits thyroid hormone activity via competition for glutathione (GSH) by both anti-oxidant enzymes and the above mentioned deiodinase enzymes (Wajner et al., 2012). Researchers believe that oxidative stress “depletes the glutathione” required for the conversion of T4 into T3, resulting in lower concentrations of active thyroid hormones.
Similarly, NTIS researchers hypothesize that the competition for and the resulting depletion of the trace mineral selenium – a component of both the deiodinase and the anti-oxidant enzymes (Wajner et al., 2015) – may amplify the link between increased oxidative stress and low thyroid hormone activity.
In sum, in the event of low thyroid hormone activity, the body is unable to make enough anti-oxidants to counteract the pro-oxidants. This leads to increased oxidative stress, which tends to depress the activity of thyroid hormones further, due to competition for glutathione and selenium in a self-perpetuating cycle (i.e. a smaller “vicious circle” is present within the larger one).
Mechanism #3: Oxidative stress stimulates pro-inflammatory cytokines
The final mechanism which completes the “vicious circle” in chronic NTIS is the link between oxidative stress and inflammation.
Oxidative stress stimulates the production of inflammatory cytokines, notably leptin, resistin, TNF-α, and IL-6 (Chatterjee, 2016).
In turn, inflammatory cytokines (notably IL-6) further increase oxidative stress by triggering the production of superoxide radicals (Valko et al., 2007; Wajner et al., 2012).
In sum, oxidative stress triggers the release of additional inflammatory cytokines which reduce thyroid hormone activity – leading to more oxidative stress. There is thus a tendency for oxidative stress and pro-inflammatory cytokines to perpetuate each other (i.e. forming yet another smaller “vicious circle” within the larger one).
Section 2: Implications of NTIS and similarities with ME/CFS
In Section 1, I provided an overview of the key mechanisms of the “vicious circle” which underpins chronic NTIS following trauma and critical illness as described by NTIS researchers:
- cytokines depress thyroid hormone activity;
- low thyroid hormone activity increases oxidative stress;
- increased oxidative stress stimulates the production of pro-inflammatory cytokines … which perpetuates the “vicious circle.”
Moreover, the research indicates that there are arrows cutting across the “vicious circle” as well. Reciprocal relationships exist between thyroid hormone status and oxidative stress; between oxidative stress and inflammation; and between oxidative stress and thyroid hormone activity. These smaller “vicious circles” within the elements of the larger “vicious circle” in chronic NTIS appear to make it even harder for patients to escape from the trap.
In the next paragraphs I will describe the implication of these mechanisms and highlight similarities with ME/CFS, in order to argue that the NTIS research can likely inform our understanding of the mechanisms underlying ME/CFS.
Implications of oxidative stress
The implications of chronic oxidative stress in the body are widely documented. Oxidative stress causes cell damage and disrupts normal cellular transcription and signaling mechanisms (Mancini et al, 2016).
Numerous studies have found increased oxidative stress in ME/CFS. Oxidative stress has been identified as a factor in the metabolic dysfunction in ME/CFS (Morris and Maes, 2014; Armstrong et al., 2015). Indeed, a model which describes a “vicious circle” involving oxidative / nitrosative stress and cytokines in ME/CFS has been proposed by Prof. Martin Pall (cf. the “NO/ONOO-Cycle,” 2010). Researchers also suggest that high lactate / low glutathione levels found in the brains of people with ME/CFS likely derive from similar mechanisms involving oxidative stress (Shungu et al., 2012)
Implications of tissue specific reduced thyroid hormone activity
One implication of prolonged reduced thyroid hormone activity seen in NTIS is, of course, a down-regulation of metabolism. Metabolic studies document a similar down-regulation in ME/CFS (Naviaux et al., 2016). Moreover, reduced mitochondrial activity has been suggested in NTIS (Warner and Beckett, 2010) and observed in ME/CFS (Myhill et al., 2009; Esfandyarpour et al., 2019).
Certainly, many ME/CFS practitioners now recognize that reduced thyroid hormone activity plays a role in ME/CFS (see my previous blog post). Ruiz-Núñez’s et al. (2018) found that CFS patients had significantly lower levels of active thyroid hormone (T3) and a higher ratio of inactivated to active thyroid hormones (rT3:T3) than controls.
Moreover, one implication of a tissue-specific down-regulation of thyroid hormone activity is that some organs will work better than others. Experimenting in rats, researchers have shown that depressed thyroid hormone levels occur in a specific sequence, manifesting (from first to last) in the liver, kidney, brain, heart and adipose tissues (Donzelli et al., 2016). Some ME/CFS practitioners have argued that this tissue-specific modulation can help explain the disparate and evolving symptoms in ME/CFS and fibromyalgia (Lowe, 2000; Lowe and Yellin, 2008; Holtorf, 2014a and Holtorf 2014b).
The prolonged down-regulation of thyroid hormone activity certainly has implications for the immune system. Authors describe the profound effects of circulating thyroid hormone levels on the activity of monocytes, lymphocytes, macrophages, neutrophils, dendritic cells and natural killer cells (Balaz et al., 1980; Pillay, 1998; Klecha et al., 2005; Klein, 2006; Hodkinson, 2009; Straub et al., 2010; Jara et al., 2017; Bilal et al., 2017; Van der Spek et al., 2018). Notably, depressed thyroid levels appear to depress the activity of natural killer cells (DeVito et al., 2011) – a signature finding in ME/CFS (Klimas et al., 1990).
Implications of altered cytokine levels and suppression of endocrine axes
The alterations in cytokines found in NTIS – notably increased markers of inflammation – likely have many additional implications that have yet to be fully understood (Van der Spek et al., 2017). Here again, parallels can be drawn between NTIS and ME/CFS: distinct cytokine signatures have been found in ME/CFS (Montoya et al., 2016) and fibromyalgia (Hernandez et al., 2010).
Finally, cytokines may be a culprit in the “central” (hypothalamic or pituitary) suppression of the growth hormone and adrenocortical hormones axes in prolonged critical illness (van den Berghen, 2016) — in addition to the suppression of the thyroid hormone axis (described in this blog post). It has been shown that adrenocortical hormone axes are suppressed in ME/CFS (see review by Tomas et al., 2013). Indeed, several ME/CFS practitioners hypothesize that the central suppression of endocrine axes is an essential mechanism underlying ME/CFS (Teitelbaum, 2007; Holtorf, 2008; Purser, 2010) — perhaps it is at the heart of both ME/CFS and prolonged critical illness?
Section 3: Emerging treatments for NTIS
Based on the increasing recognition of chronic NTIS as a “maladaptive process” following trauma or critical illness, researchers have been looking for remedies that target the different elements of the “vicious circle” described in Section 1: low active thyroid hormone status, oxidative stress, and inflammation (see review in Fliers et al., 2015).
Thyroid hormone supplementation
Given the reduced thyroid hormone activity in NTIS, clinicians began, as early as the 1980s, to suggest thyroid hormone supplementation in their critical NTIS patients in an attempt to increase their survival rates (Carter et al., 1977; Brent et al., 1986 and DeGroot, 1999). This approach continues to be debated today (Davis, 2008; Kaptein et al., 2010; De Groot, 2015; De Neto et al., 2016; Breitzig et al., 2018).
Results with thyroid supplementation have been mixed (Farwell, 2008), but have most often been beneficial (see review in Fliers et al., 2015). Interestingly, positive results have reportedly been achieved by supplementing thyroid hormones in NTIS patients who had become ill after mold exposure (Somppi, 2017).
Given the impaired conversion of T4 to T3 in NTIS, some researchers also propose T3 supplementation (as opposed to T4 supplementation) (Biondi, 2014). Moreover, tests on rabbits have shown that thyroid hormone supplementation doses have to be very high to achieve results (Debavaye, 2008). These suggestions regarding the type and quantity of thyroid hormone supplementation in NTIS echo the arguments made by some practitioners on T3 supplementation for ME/CFS (see my previous blog post).
Many publications simply conclude that more studies on the effects of thyroid hormone supplementation on tissue thyroid levels, etc. in NTIS will be required:
“Indeed, it is probable that a full understanding of the pathophysiological mechanisms at the tissue level will allow the identification of patients who would benefit from replacement therapy.” (Mancini et al., 2016).
Stimulating the hypothalamus and pituitary
Van den Berghe et al. (2002, 2014, 2016) achieved metabolic improvements using infusions of growth hormone-releasing peptide and thyrotropin-releasing hormone (TRH) in patients with protracted critical illnesses. In other words, they’ve succeeded in breaking the “vicious circle” in prolonged NTIS by stimulating the hypothalamus and pituitary to restore thyroid hormone concentrations (see “central mechanisms” in Section 1 above).
Crucially, they have, through this approach, addressed problems not just at the hypothalamus-pituitary-thyroid axis, but at other endocrine axes controlled by the hypothalamus – i.e. the hypothalamus-pituitary-growth hormone and hypothalamus-pituitary-adrenocortical axes – two axes not covered in this blog post but which are also impacted in critical illness and trauma (and work in association with the thyroid axis).
Anti-oxidant supplementation
In one case, researchers have found that treating patients of acute myocardial infarction with nacetyl-cysteine (NAC) – a precursor to the anti-oxidant GSH – could virtually eliminate the decrease in serum T3 levels and prevent the increase in serum rT3 usually seen (Vidart et al., 2014). In other words, they seem to have broken the “vicious circle” by restoring oxidative balance via the administration of anti-oxidants. (Recall that during oxidative stress, competition for GSH by both the anti-oxidant enzymes and the deiodinase enzymes leads to deranged thyroid hormone conversion as well as increases in pro-inflammatory cytokines.)
Selenium supplementation
Research suggests that supplementation with selenium is associated with modest “normalization” of thyroid hormones in NTIS patients (Berger et al., 2001). Moreover, controlled experiments on human cells showed that the administration of sodium selenite reduces cytokine (IL-6)-induced oxidative stress, but it did not fully restore thyroid hormone conversion (Wajner et al., 2016). This implies that selenium supplementation can be helpful, but alone it is insufficient to break the “vicious circle” in NTIS. (Recall that anti-oxidant enzymes and thyroid deiodinase enzymes both contain selenium, and thus this mineral is required for their production.)
Cytokine blockers
Researchers tried to stop the vicious circle in NTIS by blocking the IL-1 cytokine receptors (via a receptor antagonist), but this did not prevent the drop in T4, free T4, T3, and TSH or the rise in rT3 caused by endotoxin (van der Poll, 1995). This result is not surprising, given that “cytokines are related to each other in a very complex network and regulate, positively or negatively, the expression of other cytokines; it is, therefore, difficult to imagine how to interrupt this interplay and cascade of events” (Bartelena, 1998). (Recall that IL-1 cytokines are involved in both central and peripheral mechanisms to reduce thyroid hormone activity.)
Immune system modulation
One interesting hypothesis suggests that “it is the immune system — not the endocrine system — that re-starts the process of thyroid hormone output” during recovery from NTIS (Klein, 2006). This hypothesis is further supported by the surprising finding that immune cells are able to produce TSH, T4 and T3 on their own (Bilal et al, 2017).
Klein writes: “The immune system, which is indeed capable of TSH production, would be well suited to determine the optimal time to initiate the thyroid hormone recovery phase. A critical factor in this would be the inherent ability of the immune system to continually assess the status of the infectious condition, and thus to determine whether or not it is safe for the host to return to a state of normal metabolic activity.”
Following this logic — and recognizing the regulatory effect thyroid hormones have on the immune system — some have suggested modulating the immune functions via clinical manipulation of thyroid hormone levels (DeVito et al., 2011). Supplementation with thyroid hormones might therefore serve in at least two ways to break the “vicious circle”: directly by increasing thyroid hormone activity, and indirectly by modulating the immune system.
In sum, the overlap in treatment attempts for ME/CFS and NTIS underlines the importance of NTIS research results also being considered in relation to ME/CFS.
Conclusion:
Given the similarities between the profiles of ME/CFS and chronic NTIS following trauma and critical illness (see Table 1 below), the findings from five decades of NTIS research may also be relevant to understanding the mechanisms behind ME/CFS.
A defining feature of NTIS is low thyroid hormone activity, which in prolonged or chronic NTIS, is mediated by cytokines and oxidative stress through both “central” and “peripheral” mechanisms. The reduced plasma thyroid hormone levels readily observed in NTIS likely reflect just the “tip of the iceberg” of the thyroid hormone level reductions seen at the tissue level in these critical patients.
Research on chronic NTIS has elucidated the reciprocal relationships between cytokines, thyroid hormone activity and oxidative stress which suggest chronic NTIS patients may be stuck in a “vicious circle.”
The implications of the tissue-specific down-regulation of thyroid hormone activity on the body are numerous, including depressed immune system function.
NTIS researchers and clinicians looking for ways to break patients out of this “vicious circle” have used experimental treatments similar to those used by some ME/CFS practitioners. These include supplementation with thyroid hormones, administration of hypothalamic releasing factors, supplementation with anti-oxidants, and attempts to mitigate the effect of cytokines.
Additional research on trauma and critically ill patients describes a similar pattern of “central” (hypothalamic or pituitary) suppression of other endocrine systems not covered by this blog post (i.e. the hypothalamus-pituitary-growth hormone and hypothalamus-pituitary-adrenocortical axes). The suppression of endocrine axes at the central level echos the hypotheses of several ME/CFS practitioners.
Finally, I suggest that further exchanges between researchers working on ME/CFS and trauma and critical illness would likely greatly benefit both research communities in the quest for treatments for both NTIS and ME/CFS.
The Low T3 Series on Health Rising
- The Atypical Thyroid Issues in Chronic Fatigue Syndrome (ME/CFS), Plus a New Thyroid Subset?
- Pure T3 Thyroid and Stories of Recovery from Chronic Fatigue Syndrome (ME/CFS) and Fibromyalgia: An Overview.
The Critical Illness Series on Health Rising
- Neither dying, nor recovering”: Learning from ICUs to Solve ME/CFS and Fibromyalgia – A Synopsis (Nov. 2019)
Notes
Table 1: Similarities between NTIS and ME/CFS
The “bio-markers” or “signature” findings in ME/CFS described by recent studies are similar to findings in NTIS. The list of similarities highlighted in the table below is not exhaustive, but may be sufficient to suggest that the research into the mechanisms behind chronic NTIS is likely relevant to understanding the mechanisms behind ME/CFS.
| Findings in ME/CFS | Details for ME/CFS | Similarities in NTIS |
| Altered immune cell activity | Klimas et al. (1990) and many others have found reduced Natural Killer cell function in ME/CFS patients. | A prolonged deficiency of thyroid hormone is related to the impairment of the cellular immune system (Pillay, 1998; Van der Spek et al., 2017) – notably reduced activity of natural killer cells (DeVito et al., 2011). |
| Down-regulation of metabolism
|
Naviaux et al. (2016) found irregularities in the metabolites of ME/CFS patients, “consistent with a hypometabolic syndrome.” | NTIS is characterized by low active thyroid hormone inducing a generalized hypo-metabolic state (Warner and Beckett, 2010; Wajner et al., 2012). |
| Reduced mitochondrial activity | Myhill et al. (2009) showed that ME/CFS patients were unable to replenish ATP stores, attributing this to reduced mitochondrial activity. See also Esfandyarpour et al. (2019). | Reduced intra-cellular ATP has also been suggested as a mechanism in NTIS (Warner and Beckett, 2010). |
| Distinct cytokine signatures
|
Montoya et. al (2017) found that some 17 cytokines were positively correlated with the severity of ME/CFS, of which 13 are pro-inflammatory (see also Hornig et al., 2015). Similarly, circulatory levels of proinflammatory cytokines are altered in fibromyalgia patients (Hernandez et al., 2010). | NTIS has long been associated with changes in cytokines (Boelen et al., 1993; Bartelena et al., 1998). |
| Oxidative Stress | ME/CFS has been associated with oxidative stress (Morris and Maes, 2014; Armstrong et al., 2015). | Oxidative stress is a key mechanism in NTIS (see Wajner et al., 2012; Mancini et. al, 2016 and Chatzitomaris et al., 2017). |
| Reduced plasma levels of active thyroid hormone; increased levels of biological inactivated thyroid hormones | Ruiz-Núñez’s et al. (2018) found that, as a group, CFS patients had significantly lower “Total T3” (TT3) levels (i.e. the active form of thyroid hormones) and a higher “rT3 to total T3 ratio” (rT3:TT3) than controls – meaning a higher ratio of inactivated to active thyroid hormones. They write: “low circulating T3 and the apparent shift from T3 to rT3 may reflect more severely depressed tissue T3 levels.” | NTIS is by definition a reduction in plasma levels of active thyroid hormone. (Chopra, 1979; Warner and Beckett, 2010). The decrease in T3/rT3 relationship is considered the most sensitive parameter for diagnosis of NTIS (Neto et al., 2016). |
| Dysregulation in adrenal hormones | Di Giorgio et al. (2016) have found that the hypothalamus- pituitary- adrenal axis is dysregulated in ME/CFS patients (see also: Teitelbaum, 1996; Durrant-Peatfield, 2006; Skinner, 2003, Tomas et al., 2013). | “Prolonged NTIS is hallmarked by a uniform suppression of the neuroendocrine axes, predominantly of central/hypothalamic origin, which contributes to the low (or insufficiently high) circulating levels of hormones” (van den Berghe, 2016). |
| Various triggers | Chu et al. (2019) cites the most common peri-onset events reported by subjects were infection-related episodes (64%), stressful incidents (39%), and exposure to environmental toxins (20%). | NTIS occurs in response to virtually any illness or surgical stress (De Groot, 1999; Wajner et al., 2012). |
This work is licensed under a Creative Commons Attribution 4.0 International License.









Hello, I have long-term ME/CFS. Recent blood work with a new doctor showed that my thyroid hormone levels are normal but my iodine level is extremely low as is my Vit. D level. My doctor says that as a result I am unable to utilise my thyroid hormones mimicking hypothyroidism. I would be interested in your response to this. Thank you,
Dear Jane,
Thank you for your comment! I found the websites of patient advocates very useful for information on minerals and vitamins for improving the functioning of the thyroid hormone. Check out the websites: RT3 adrenals, Stop the Thyroid Madness, Thyroid Patient Advocacy UK and Hypothyroid Mom. I also found the book by Dr Barry Peatfield-Durrant very informative. Please also check out my first blog post on some approaches to treating depressed thyroid hormone function in ME/CFS.
Best wishes!
Dominic
Interesting, I was just reading, most brain cells are immune cells, and then inflamed brain cells diminish function, obviously.
Great piece. I was diagnosed with both subclinical hypothyroidism and ME/CFS roughly 14 years ago, and have been on T3/T4 treatment since. In those years whenever I had a EBV reactivation my thyroid would become severely suppressed. But even in “normal” times, T4 alone did absolutely nothing for me. The addition of T3 (in the form of Armor Thyroid) made a large difference, but not in my ME symptoms. It helped relieve the fatigue and other symptoms from the hypothyroidism, but I’m still disabled with ME. That said, I’d be entirely incapacitated if I was not on thyroid supplementation. It had a large impact on my overall wellbeing. I also make sure I get enough nutritional iodine and selenium from foods and add supplemental glutathione.
That said, a thyroid test called the TRH is not used often these days, but I believe it can test for secondary forms of thyroid malfunction not originating from the thyroid itself (hypothalamus, etc). Often subclinical or normal TSH ranges are found to have abnormal TRH test results. Sadly this test is impossible to find these days. I feel like it could be a beneficial tool in treating secondary and tertiary forms of thyroid disease, whether caused by inflammation or otherwise. It’s how I got on the right track years back. Still stuck in a metabolic trap though. But at least I’m not in a severe ME phase anymore, which I was at the time of diagnosis. Perhaps looking at more investigative testing can help lead to more clues about thyroid health in patients across the board.
Dear Mikki,
Thanks for your comment. And good to hear that thyroid hormone treatment with T3 relieved fatigue. In my first blog post I gave an overview of thyroid hormone treatment approaches with T3 for ME/CFS. What I learnt is that there are many variables that affect the treatment (quantity of hormones, ratio of T3 to T4, form of hormone, timing, complementary minerals/vitamins, etc.) and its not easy to get it optimal. There are many forums where patients discuss their experiences.
I don’t know much about the THR test, except that it can check the status of the pituitary (i.e. if it responds to the signals sent by the hypothalamus). I was interested to learn that some researchers trialed and suggest using THR to relieve depressed endocrine function in prolonged critical illness — including depressed thyroid hormone activity (van den Berghe, 2016).
Best wishes!
Dominic
Re: selenium and glutathione; food sources:
from webmed:
“”Glutathione is another nutrient that has been found to strengthen the immune system so it can fight infections. This powerful antioxidant is most plentiful in the red, pulpy area of the watermelon near the rind. It can also be found in broccoli, Brussels sprouts, cabbage, cauliflower, spinach, and other cruciferous vegetables””
“”A single Brazil nut contains 68 to 91 micrograms (mcg) of selenium, meaning that just one nut per day can provide the daily recommended adult allowance of 55 mcg. In addition to selenium, Brazil nuts contain plenty of protein, essential minerals, and healthful fats.Apr 17, 2019
https://www.medicalnewstoday.com › …
Brazil nuts: Health benefits, nutrition, and risks – Medical News Today””
Dear Sunie. Thanks for your interest and the links to the food sources for gluthatione and selenium. Best wishes! Dominic
True, but unfortunately also, from wikipedia, glutathione:
“Bioavailability and supplementation
Systemic bioavailability of orally consumed glutathione is poor because the tripeptide, is the substrate of proteases (peptidases) of the alimentary canal, and due to the absence of a specific carrier of glutathione at the level of cell membrane.[31][32]
Because direct supplementation of glutathione is not always successful, supply of the raw nutritional materials used to generate GSH, such as cysteine and glycine, may be more effective at increasing glutathione levels. ”
Many of us have poor gut functioning on top of that.
Thanks for writing this clear and well structured informative blog. Food for thought.
Thank you, Dejurgen, for your positive feedback! It took me a long time to research and write this blog post — so I really appreciate your comment! If you liked this one, please also check out my first blog post. Like many others I’m trying to bring attention to “altered thyroid hormone function” as a mechanism in ME/CFS.
Best wishes!
Dominic
Hi Dominic,
Had nothing useful to add to the blog so didn’t wanted to post meaningless things. But having few comments on it can give you the impression few valuate your precious effort, while the opposite is true. It’s like a good teacher getting few questions as all is clear.
That said, I start to think that in many cases of ME and in parallel maybe also with NITS, problems should self resolve IF no underlying issues are present. But that doesn’t mean that breaking the vicious circle is always “wrong”.
I’m a numbers person, so let me use a numerical example to explain my thoughts.
Let us take an example where a seemingly healthy person has half a gut problem. Meaning that if the problem was 100% in stead of 50% in magnitude, it should start a self reinforcing inhibition circle. Other problems like a glycogen storage disorder will do too in this hypothesis.
Now add a hit-and-run disease, a whiplash, strong stress at work…
Then temporary the total inhibition-provoking health problems reach 110, 120%… of its trigger point. Inhibition starts to kick in and put the body in a self protect mode. Inhibition will only “self resolve” once the bulk of the perceived problem is resolved. For example it only will release its grip if the total sum of health problems drops below 60%. This approach is commonly used in engineering and referred to as hysteresis. It makes a system acting more stable. When the hit-and-run disappears in a person with no other significant (often hidden) health problems the temporary inhibition unlocks and the person only experiences a temporary flu like fatigue.
But the (side effects of) inhibition itself with reduced blood flow, exercise intolerance, rounds of hypoxia at night… are behaving like a small disease in itself. Lets give it 25% of a full problem on that same scale. Then after the hit and run you still have like 50 + 25 = 75% of a health problem remaining. As it is more then the 60% needed to clear the alarm / inhibition phase the inhibition stays on.
What’s “new” on this idea? Trying to unlock Inhibition remaining on after a hit-and-run hasn’t to be either wright nor wrong. There may be many in-betweens and additional options.
In the example case clearing the inhibition with an appropriate drug would be fine, but using the opportunities to resolve enduring “background” health problems first would be a wise thing to do and self clear the inhibition.
But there are other variants:
* the health cost of inhibition is great enough to make clearing at once difficult. For example the accumulated health cost of inhibition itself is too big for the body at once to deal with. Then clearing the lingering side effects of inhibition wont happen at once by using a drug that unlocks the inhibition at once. This drug in fact could cause the body to be overwhelmed by the stacked problems, much like what would happen with releasing entire deposits of heavy metal at once.
That may be what makes Dr. Lowes approach of slowly increasing doses a bit more successful. At the same time it would be some sort of a test if the inhibition can be safely overridden but only if one not only checks for signs of over stimulation during the slow ramp up of doses, but even more so for a gradual reduction in NIST symptoms itself. Something similar should be considered future with drugs “unlocking” ME inhibition in my opinion. Starting doses and gradual increases should be really low in this approach.
Better investigating what problems inhibition itself cause and what part of them tends to linger after inhibition is shut down may be helpful here too.
People being stuck in inhibition may also reveal another underlying problem: low self clearing rates of accumulated wastes and other things. Then inhibition may create more damage a day then the self healing capabilities of the body are able to clear up. That likely would show in the slowly increasing health problems in the gradual onset cases. These would get worse and worse until inhibition is that strong that problems and waste accumulation of daily activities and inhibition itself start to slow down and match the also reduced rate of self healing. That would be at a really low level of health.
Researching what makes up self healing, a necessary thing to keep healthy people alive for even a single week, may be an overlooked aspect in trying to break people out of ME.
Thanks Dejurgen!
A few things come to mind:
– The team at King’s College in London (Russel et al. 2018) could predict which patients would develop ME/CFS-like symptoms based on the reactivity of their immune system even before being exposed to a stressor (IFN-α). So, yes, the “baseline” you are starting at matters. This may explain why some patients pass from acute NTIS into recovery, while others get stuck in prolonged NTIS.
– I also recall research which shows that childhood trauma increases the risk of CFS between 6- and 8-fold (see Tomas et al. 2013). There may also be genetic predispositions to developing ME/CFS. So the “baseline” may not be something that we can always heal easily?
– There is a series of fascinating ME/CFS papers that I think you would really like that model the HPA axis (and HPG axis). These are similar to what you describe. In sum, the models indicate that the HPA axis has 2 steady states: a low one and a high one. They are connected by a S-like curve, so the states act like attractors (you will tend towards one or the other and get “stuck” there). The models then describe what event would get a person from one state to the next (e.g. a forced drop in ACTH levels by 30% from baseline; inhibition of cytokines combined with inhibition of glucocorticoid receptors; a powerful external stressor; etc.). I was just looking into these this week. Here some of the titles:
*2018 -Sedghamiz – High-fidelity discrete modeling of the HPA axis: a study of regulatory plasticity in biology
*2015 – Craddock – Achieving Remission in Gulf War Illness: A Simulation-Based Approach to Treatment Design
*2015 – Hosseinichimeh – Modeling the hypothalamus-pituitary-adrenal axis: A review and extension
*2013 – Craddock – A Role for Homeostatic Drive in the Perpetuation of Complex Chronic Illness: Gulf War Illness and Chronic Fatigue Syndrome
*2009 – Ben Zvi – Model-Based Therapeutic Correction of Hypothalamic- Pituitary-Adrenal Axis Dysfunction
I think this numerical modelling of ME/CFS is fascinating. However, I haven’t seen a model that also includes the HPT axis. I think if we understand the interactions between the different endocrine systems (and the immune system) then we would understand ME/CFS… But I hope we can find a cure before we understand it all!! Best wishes! Dominic
This is really good info. I have had a lot of help from many of these strategies. A couple of comments and a couple of questions:
– glutathione is under stress from many angles in ME/CFS, from getting rid of toxins like heavy metals and mycotoxins, infections, and from mitochondria. Many of us cannot get enough from food. And, though NAC is helpful, many are missing glycine, or even glutamine, the other 2 aminos needed for glutathione, or B6 or other Bs used in methylation, a precursor to glutathione, or vitamin C, used to recycle glutathione.
– this oxidative (and nitrosative) stress can create peroxynitrites, as mentioned above, which can impair mito complex I, and damage mito membranes, especially if one cannot make sufficient MnSOD. Lipid replenishment of membranes as Garth Nicolson has found, seems to help repair them.
– the American Society of Endocrinologists wrongly bases all recommendations on the TSH, a hormone made by the pituitary, and many endocrinologists won’t treat if your TSH is normal, and many won’t prescribe T3. So, we either have to convince our docs that we have a pdoblem as described above, or find a doctor who is open to new ideas.
– many experts at this say that treating adrenals first is essential – many of us have found benefit from replacement dose hydrocortisone.
– optimizing thyroid is important, but unless we attend to the other angles (such as those causing the oxidative stress and cytokine issues, among others), it is difficult to get well.
And my questions:
– How does one measure tissue thyroid hormones? (I need a lot of T3 and T4 and do best when T3 is slightly above the top end of normal, I can’t function otherwise – I suspect this is the problem…)
– How might folate (5-MTHF)play into the above? My doctor recently suggested taking more 5-MTHF, which I did, 20 min after taking T3, and ehen I did, within 15 minutes my braun fog cleared and I felt energized. RBC folate was high normal and homocysteine was 6.1, so it didn’t seem like I shoukd need it. Any idea what’s going on?
Thank you!
Dear Learner1,
Thank you for your insightful comments on co-factors for glutathione production and lipid replenishment to repair damage to cell membranes from oxidative and nitrosative stress.
Please also check out my first blog post in which I gave an overview of practitioners’ approaches to recognising and treating depressed thyroid hormone activity — even when thyroid blood tests are in normal range. It seems like more and more practitioners accept that the “simple-minded reliance on laboratory tests” (quoting Dr Skinner) is cause of vast under-diagnosis of patients who have depressed thyroid hormone activity??
Regarding your question on how to measure tissue thyroid hormone levels: I think you need tissue samples. In the Donzelli et al., 2015, the rats were sacrificed to measure the levels in different tissues. I read that critical illness research also relies on post-mortem analyses for assessing endocrine function in critical ill patients. I also know that 24 Hour urine tests are used to see how much T3 tissues actually consume (as opposed to what is available in the blood which doesn’t tell us if its getting into cells and being received by receptors).
Finally, on why folate supplements helps with brain fog: I don’t know — but its very interesting. Of course, mineral/vitamin status plays a role in many of the mechanisms that determine thyroid hormone activity at tissue level. I wish we had a comprehensive guidebook on how to get optimize thyroid hormone activity. The medical community has failed patients on this in a big way. Patients themselves have tried to fill the gap with websites such as: RT3 adrenals, Stop the Thyroid Madness, Thyroid Patient Advocacy UK and Hypothyroid Mom. (See full list of resources in my first blog post).
Best wishes!
Dominic
hi Learner,
“vitamin C, used to recycle glutathione.”
Vitamin C is actually recycled by glutathione, but as both can scavenge ROS, supplementing with vitamin C can reduce the strain on glutathione a bit. Without enough glutathione (and NADPH for recycling glutathione), vitamin C becomes a use once (actually twice as it can scavenge two times ROS) and discard supplement. That in effect makes supplementing vitamin C still helpful but to a far lesser extent (per grams supplemented) then when the body can recycle sufficient glutathione in order to recycle vitamin C.
“many of us have found benefit from replacement dose hydrocortisone”
cortisol , similar to cortisone , nor-adrenaline, adrenaline and thyroid hormones can increase energy production.
That may be their point of action: by increasing cellular energy production you override inhibition. That could work in some cases and effectiveness of it could increase if patients paced like if not taking these for a prolonged time. E.g. like using only 20% of the increase in energy levels and allow the remaining 80% to be used by the body for better healing and self repair. Problem is, these hormones are also activating hormones.
They entice many patients to use near 100 and often over 100% of the increase in energy to do more. When the body started inhibition in order to allow it to heal, then that is a recipe for disaster in the long run IMO.
Just using “only” 80% of improved energy doesn’t leave 20% on the table to improve self healing neither as supplementing these hormones itself does have a cost too.
Taking a numerical example of the hormones (nor-)adrenaline, a hormone many ME patients self produce.
When levels are high, patients feel energized. Let us assume a patient feels an increase of 60% in energy and abilities due to the surge. Even in the case the patient is pacing well and only uses 2/3th or 40% more energy to do activities, there are still the many side effects of boost in these stress hormones. If (most of) these costs only start to kick in half a day later then the patient feels better, he has 40% more energy and 20% more energy to self heal. Great! Top of the world! More energy! Less pain!
Then, side effects start to kick in. Like being 2/3th of the original boost in energy. But having already spend 2/3th of it, not so much is left. Crash follows in a delayed fashion as now you have !less! energy available for activities and self-healing. Result: you feel miserable having less energy and having less self-healing capacity. If the initial boost in adrenaline is over, you’re down to 60% of original pre-boost energy levels for combined activities and self-healing. That will hurt, literally as your cells are overrun by delayed onset ROS.
Often, things are worse as this happens during night when energy hormones are already low. Big crash! Expect another midnight adrenaline surge to save you from the worst. That’s bad sleep and still feeling like being driven over by a car.
Point of the story: when trying to boost energy producing hormones or having a spontaneous one, try to moderate it and prefer length of a moderate boost over a strong surge. AND only ever use 20% of the increase for activities so long as not being healed. That is a very difficult one including for me to follow!
This is such a well written piece describing complex interactions that might keep people with ME/CFS ill. My doctor didn’t explain why he prescribed 3 different types of thyroid supplementation, but I’m glad he did. I still don’t have a lot of energy, but I think I would feel much more ill if I wasn’t taking T3, Armour thyroid (T3 & T4), plus Synthroid (T4 only). Thank you for your fine explanations of the vicious circle and how one might intervene.
Thank you, Sarah, for your positive feedback!
Please also check out my first blog post in which I gave an overview of practitioners’ approaches to treating depressed thyroid hormone activity with various thyroid hormone supplementations. I hadn’t heard of a doctor mixing all of the 3 main types, but if it works that’s great! Perhaps your doctor’s approach is an innovative way to allow easier optimization of the quantities and ratio of T3 and T4 over time?
Best wishes!
Dominic
About a year ago I went back to the only physician who ever made me feel better. I received my Fibromyagia diagnosis first in 2007. 5 years later I was diagnosed with ME/CFS in 2012 and received a course of Acyclovir for over 6 weeks and felt better. My endocrinologist at the time prescribed hydrocortisol and L-Thyroxine.
Last year after several years with only L-Thyroxine treatment I felt so miserable I returned to my endocrinologist. My cortisol levels were non-existent and the T4 wasn’t converting to T3. So I was put back on hydrocortisol (25 mg spread during a normal not active day – mostly the Rose is increased), the L-Thyroxine was lowered to 100 micro-grams and I was put on L-T3 (25 micrograms). I do feel better but the ME/CFS symptoms aren’t gone.
Although it makes a remarkable difference!
Dear Invisible,
Thanks for your comment.
Please also check out my first blog post in which I gave an overview of practitioners’ approaches to treating depressed thyroid hormone activity with various thyroid hormone supplementations. I learnt that there are a lot of variables involved in optimizing thyroid hormone supplementation (quantity of hormones, ratio of T3 to T4, form of hormone, timing, frequency, complementary minerals/vitamins, etc.) and its not easy to get it optimal. In my first blog I also provide a list of forums where patients exchange on this treatment. I am glad to hear you already had some real improvement with it! (and the anti-virals). Best wishes! Dominic
Is there ANY doctor or other “practitioner” who — FIRST — investigates whether the individual has enough actual IODINE ? Iodine in their body, in their diet ?
The FIRST action should never be thyroid HORMONE. Has the world forgotten that iodine is necessary, first ?
This is incredibly well written and researched. Thanks for putting this together.
Thank you Brian! Please also check out my first blog post. And share them both widely! Thanks. Best wishes! Dominic
Nice! Thank you for sharing this, Dominic.
I feel the best on a combination of Armour Thyroid and Standard Process Thytrophin PMG (a T1, T2 and T3 product).
I was taking iodine, but I overdid it and gave myself a goiter. Now I am re-balancing myself with only Kelp as a source of iodine and more Armour.
I take selenium too. I took tiny amounts of Cortef for a few years, but now I take DHEA because it is gentler. I no longer need the really strong adrenal support of cortisol.
It would sure be nice to be able to test TRH. My TSH level is going down as I age, and it has happened to my mom too. She does not have ME/CFS.
What a lot of top-class investigation and writing – makes real sense of a puzzling illness. My wife has had hypothyroidism then ME for over 25 years, and only recently discovered RT3 – and high levels on three checks. I’m ashamed to have worked for the NHS for 40 years, and have to do much of our tests privately, and get more help from these forums than the medical services. The low T3 syndrome covers our test neatly. But precise mitochondrial physiology needs more ‘proper science’. As the Bible says, we are wonderfully made. Very many thanks for giving us real hope. Mike
Dear Mike.
Thank you for your positive feedback!
Please also check out my first blog post in which I summarise the approaches of a number of physicians treating with T3. And please share my post with others to raise awareness.
Thank you and best wishes!
Dominic
Great article!!! Thank you so much. I was wondering about the part on the Immune system modulation. Do you think this could be a reason that LDN helps with some patients. I understand it effects the thyroid and that it is an immune modulated?
Thanks again for all the time that you have put into your two blogs on the thyroid.
Dear Phatluvskier,
Thank you for your positive feedback!
I imagine that that is why LDN’s immune modulation effect may help, but I honestly don’t know. Its an interesting question. I’ll look if I find something on LDN and the cytokines involved in NTIS! (But there are so many cytokines and their interactions amongst themselves are so complex, that probably nobody really knows!)
Best wishes!
Dominic