

Check out Geoff’s Narration
The GIST
The Blog
A Deep Dive into the Muscles Pays Off
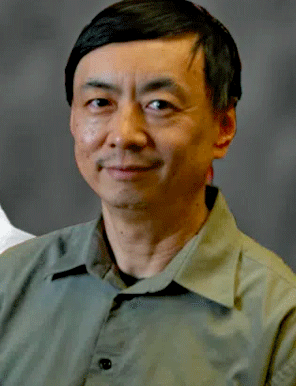
A leader in computational analysis, Wengzhong Xiao, of the Open Medicine Foundation, co-led the study.
It’s so good to see the muscles getting more research in chronic fatigue syndrome (ME/CFS). Rob Wust’s recent long-COVID study exploring the effects of exercise on the muscles reaped dividends, and right on the heels of that, we have a very different dive into them – and this one suggested a treatment possibility.
Note that both these studies were largely funded by patient organizations: Wust’s study by the Solve ME/CFS Initiative, and the Patient-Led Research Collaborative for Long COVID, and the Xiao co-led study, below, by the Open Medicine Foundation.
Patient organizations are great at getting ahead of the curve and opening up new insights and even new fields of inquiry. They boldly go where the big funders like the NIH won’t, and in these two cases (and others), they hit paydirt. We should expect more on these topics in the future.
THE GIST
- It’s so good to see the muscles getting more research in chronic fatigue syndrome (ME/CFS). Rob Wust’s recent long-COVID study, exploring the effects of exercise on the muscles, reaped dividends, and right on the heels of that, we have a very different dive into the muscles – and this one even suggested a treatment possibility.
- Note that both these studies were largely funded by patient organizations: Wust’s study by the Solve ME/CFS Initiative, and the Patient-Led Research Collaborative for Long COVID, and the Xiao co-led study, below, by the Open Medicine Foundation. Exploring and opening up ground in new topics is what patient organizations do best. They play a crucial role in ME/CFS research.
- The “Systems Modeling Reveals Shared Metabolic Dysregulation and Novel Therapeutic Treatments in ME/CFS and Long COVID” study, co-led by the Open Medicine Foundation’s Wenzhong Xiao, employed advanced analytic techniques to explore what’s going on in the muscles.
- The researchers integrated gene expression, proteins and “kinetome” data to predict the metabolic networks at play in the muscles of people with ME/CFS. Those networks work together to produce energy and the essential building blocks of the body (proteins, fats, nucleic acids and some carbohydrates). If something is awry with energy production, it may very well show up in our metabolic studies, and these kinds of studies have, indeed, proven invaluable in ME/CFS.
- The findings highlighted six metabolic pathways, the most prominent of which was a downregulation of the alanine and aspartate metabolism pathway. A comparative analysis of Rob Wust’s long-COVID muscle study concurred with the result of the ME/CFS study.
- They concluded that “a fundamental disruption in amino acid metabolism and energy metabolism” involving, in particular, the breakdown and conversion of alanine and aspartate has occurred in both ME/CFS and long COVID.
- The low alanine finding had a startling tie-in with both ME/CFS and long COVID: alanine plays a key role in getting rid of ammonia, and studies suggest that ammonia – a byproduct of anaerobic energy production – may be building up in the bodies of ME/CFS patients and causing fatigue, brain fog, etc.
- The authors proposed that using the L-ornithine plus L-aspartate (LOLA) supplement regimen “could potentially rescue the metabolic changes observed in ME/CFS patients” (!). LOLA is a supplement combination that is readily available and has been used for decades to reduce ammonia levels in people with liver problems. That’s intriguing given that some researchers believe the liver is involved in ME/CFS. (See Kalafatis’s recovery story).
- Note that the authors do not suggest that L-alanine and L-ornithine supplementation will cure ME/CFS. They suggest that doing so “could potentially mitigate some of the core symptoms”; i.e. make less severe some of the core symptoms.
- L-aspartate has been used to increase endurance and reduce fatigue. It has also been shown to decrease lactate and increase fatty acid oxidation – which appears to be impaired in ME/CFS. It may also be able to increase nitric oxide levels, improve blood flows, and reduce ammonia levels. L-ornithine helps eliminate ammonia and fatigue-causing metabolites in the muscles.
- Given the current interest in oxaloacetate in ME/CFS and long COVID, L-aspartate, interestingly, is converted into oxaloacetate outside the mitochondria, and once inside the mitochondria, oxaloacetate is converted back into aspartate. One wonders if using LOLA in combination with oxaloacetate could be helpful.
- In conclusion, the study was too small (25 MECFS patients) for us to assume that it was representative, but the fact that its core findings were validated in separate tests within the ME/CFS group, and then in the long-COVID group, suggests that it might very well have legs. LOLA’s known ability to remove ammonia, possibly improve endurance and blood flows, and liver functioning as well, and its connection to oxaloacetate makes it an intriguing substance indeed.
- Congratulations to the Open Medicine Foundation for helping to fund this study.
Metabolic Dysregulation and Treatment Possibilities?
The “Systems Modeling Reveals Shared Metabolic Dysregulation and Novel Therapeutic Treatments in ME/CFS and Long COVID” study was led by the Open Medicine Foundation’s Wenzhong Xiao and researchers from the Laboratory of Genetic Evolution & Animal Models (Chinese Academy of Sciences). It was not large – included 13 ME/CFS patients and 12 healthy controls (as well as an outside long COVID group in part of the study) – but it employed advanced analytic techniques and most importantly, took a deep dive in the muscles.
The researchers used a cutting-edge modeling technique called “genome-wide precision metabolic modeling (GPMM)” that integrates gene expression, proteins and “kinetome” data to predict what metabolic networks are involved. In other words, this technique is able to do just what we want to do with these large datasets – bring all the factors together.
It was no surprise to see Wenzhong Xiao, the senior author, lead this type of study. The Co-Director of the Open Medicine Foundation’s Ronald G. Tompkins Harvard ME/CFS Collaboration at the Harvard Affiliated Hospitals and Director of the Immuno-Metabolic Computational Center at Massachusetts General Hospital (MGH), Harvard Medical School, Xiao’s main focus is on bringing big datasets together to better understand illnesses.
Note that this study was all about metabolism – it used modeling techniques to determine how multiple factors (gene expression, proteins, kinetome) affected the metabolic networks in the body. Those networks work together to produce energy and the essential building blocks of the body (proteins, fats, nucleic acids and some carbohydrates). If something is awry with energy production it may very well show up in our metabolism. Indeed, over the past ten years or so, metabolomic studies have gained more and more prominence in ME/CFS.
The study also demonstrated the importance of open data: it used muscle data from Nath’s intramural study to assess the metabolic networks involved. Then they compared those findings to data from Rob Wust’s recent long-COVID muscle study to determine how similar ME/CFS and long COVID were.
Results
Chronic Fatigue Syndrome (ME/CFS)
The findings highlighted six metabolic pathways (downregulated: alanine and aspartate metabolism, pyrimidine catabolism, aminosugar metabolism, arginine and proline metabolism pathways; upregulated: pentose phosphate) with the alanine and aspartate metabolism pathway being the most downregulated.
Then they did an “all-knockout analysis”, which perhaps refers to something like the pathways being matched against each other pathway until the most significant pathway emerged (???). In any case, the analysis highlighted the alanine and aspartate as the most prominent “agonist” metabolites; i.e. metabolites, if I’m reading it right, that would most enhance the metabolism in ME/CFS.
Long COVID
Next, they assessed the metabolic changes from pre to post-exercise in Rob Wust’s illuminating long-COVID study – and found that asparagine was the most downregulated amino acid metabolite in both blood and muscle samples.
ME/CFS and Long COVID
The authors concluded that “a fundamental disruption in amino acid metabolism and energy metabolism” has occurred in both ME/CF and long COVID. The most significant metabolic alteration found involved the breakdown and conversion of alanine and aspartate.
Indications that something is up with alanine have showed up a couple of times in ME/CFS. McGregor’s 1996 “Preliminary determination of the association between symptom expression and urinary metabolites in subjects with chronic fatigue syndrome” study proposed that beta-alanine (and an unknown metabolite) might “provide a molecular basis for developing an objective test for CFS”. In 2006, his “Preliminary determination of a molecular basis of chronic fatigue syndrome” singled out reductions in alanine as a key factor in ME/CFS. Reductions in gut alanine levels showed up in a recent retrospective of gut microbiome studies. A 2022 mass spectrometry study found alterations of beta-alanine (and arginine and proline metabolism).
Effects

Chris Armstrong is checking whether higher than normal levels of ammonia are being produced in ME/CFS.
What do these two amino acids do in the body? Alanine traps and scavenges ammonia and plays a role in the TCA cycle in the mitochondria. The dramatic reduction in alanine found in ME/CFS suggested that the ammonia removal process had been impaired.
That could be a particularly big deal for these diseases. Study after study has shown that amino acids are being preferentially used in ME/CFS to produce energy. Amino acids, though, have a pesky nitrogen atom attached to them that needs to be taken care of.
The body usually eliminates the nitrogen from amino acids using a variety of “safe” forms, but those safe forms have been found wanting in ME/CFS metabolomic studies. Chris Armstrong – who leads the Australia Open Medicine Foundation Research Center – believes the nitrogen byproducts of amino acid breakdown may be showing up as “unsafe” forms such as ammonia or peroxynitrite in ME/CFS.
There’s, of course, nothing good about increased ammonia levels in the body. It inhibits cell growth and damages cells and generally mucks things up. Armstrong is tracking cells from ME/CFS patients to determine if this is so.
One wonders if the toxic feeling that some people experience is, in part, due to increased ammonia levels? In another possible tie-in, the liver is a major source of ammonia metabolism and several researchers believe that liver issues may play a role in ME/CFS as well.

Could LOLA supplementation help “rescue” the metabolic problems in ME/CFS and long COVID?
Treatment Suggestion
The authors proposed that “Administering these two amino acids (LOLA – see below) could potentially rescue the metabolic changes observed in ME/CFS patients” (!). This is the first metabolic study I can remember that suggested a specific treatment. (A metabolomic study did lead to Dr. Kaufman’s use of oxaloacetate, but he gloomed onto that supplement after seeing low oxaloacetate results to find it.)
LOLA
LOLA is the term used for the L-ornithine L-aspartate supplement. LOLA has been used to reduce ammonia levels in non-alcoholic fatty liver disease for decades. Note that the authors do not suggest that L-alanine and L-ornithine supplementation will cure ME/CFS. Let’s not put that on them. They suggest that doing so “could potentially mitigate some of the core symptoms”; i.e. make less severe some of the core symptoms.
In the U.S. the L-ornithine L-aspartate (LOLA) supplement mix is available in powder or pill form on Amazon. (Health Rising does not benefit from Amazon sales.
One wonders, though, how they might work with oxaloacetate – given its very close connection with L-aspartate (see below) – and other mitochondrial supplements, or in conjunction with the liver supplementation regimen that
Check out what the two components in LOLA (L-ornithine L-aspartate) can do.
L-aspartate
L-aspartate would enhance the ASN/ASP pathway. One website stated that L-aspartate is being used to increase muscle strength, improve sports performance, and reduce fatigue. One study found while L-aspartate did not increase power, it did appear to increase endurance during repeated sprints. It has also been shown to decrease lactate and increase fatty acid oxidation – which appears to be impaired in ME/CFS. It may also be able to increase nitric oxide levels and improve blood flows.
Given the current interest in oxaloacetate in ME/CFS and long COVID, L-aspartate, interestingly, is converted into oxaloacetate outside the mitochondria, and once inside the mitochondria, oxaloacetate is converted back into aspartate. It also helps with the removal of ammonia. It also may be able to enhance the production of testosterone.
L-aspartate supplementation can improve energy levels, help remove ammonia, increase muscle mass, improve liver functioning and help with atherosclerosis. It can also produce side effects like bloating and diarrhea.
As always, best to start low and go slow.
L-ornithine
Adding L-ornithine could enhance the downregulated arginine and proline metabolism pathways, and help eliminate ammonia by turning it into urea, which is then disposed of in the urine. As one site puts it, L-ornithine “helps remove bodily wastes and fatigue-causing metabolites in the muscles”. L-ornithine may also be able to promote lipid metabolism – an area of growing interest in ME/CFS. The same study suggested it can help with fatigue as well.
No side effects were mentioned.
Conclusion
In conclusion, the study was too small for us to assume that it was representative, but the fact that its core findings were validated in separate tests within the ME/CFS group and then in the long-COVID group suggests that it might very well have legs. LOLA’s known ability to remove ammonia, possibly improve endurance and blood flows, and liver functioning as well, and its connection to oxaloacetate makes it an intriguing substance indeed.
- For more on the muscles and ME/CFS check out a blog I recently wrote “Getting their due – the muscles in ME/CFS” for ME Research UK.
- Congrats to the Open Medicine Foundation (and the other funders) for supporting a fascinating study.






Interesting. Will have to read the paper. Do they theorise a causative mechanism for these observations? How do these things link to immunity etc?
Might give LOLA a try for my 16 year old daughter.
I haven’t read the paper yet but Akiko commented on the Wust findings saying that she predicted B cell involvement and I think they also found T cells happening.
This kind of study is crunching numbers / chemical/metabolite counts which we can think of as part of a cycle of derangement that also includes autoimmune mechanisms attacking muscle fibers, endothelial cells, mitochondria, autonomic nerves/receptors (see “channell patchy”) . I feel like these must feed each other in vicious cycles with exact tissues/circuits/receptors involved varying per patient/subtype.
I wonder how this study matches my recent tests on DNA & Urea levels? I have MTHFR 2 types heterozygous one is C677T I am deficient in FOLATE 4.8 insufficient in VitD
I did the 23andme health ancestry test and then downloaded all the Raw data to Free genetic genie Alport Syndrome homozygous, 2 types of mitochondria disease MT-ND4 tied to Leber Hereditary Optic Neuropathy & MT-ND5 can also be a part of Leber (LHON) or Leigh Syndrome.
Alport & Leber ears/eyes issues even blindness, Alport Syndrome 2nd leading (CKD) Chronic Kidney Disease, also has kidney failure/transplant. My urea in blood UK range was at 10 recently at 9.3
I need eye Surgery short sited-long sited cataracts lense issues, there is an eye medicine for Leber I think it is a genetic protein pill only prescription is available in Wales Belfast Scotland not in England areas
This is the first study that has suggested a mechanism for that “poisoned feeling” many patients with ME/CFS have reported over the years. While it doesn’t define a cause, the study authors do offer the possibility of a treatment that is widely available and not prohibitively expensive. That is encouraging.
Where can I get LOLA?
Which country are you in? I couldn’t find a known one in the UK and ended up getting bulk powders from USA. Just ordered today
I’m in the US.
I found it on amazon in the UK.
Kim, I would be interested to know which one you got. I found some at a ridiculous price. I also found one from a supplier I had never heard of. In the end I decided it was better to get it from a supplier in the USA that friends had used before. It worked out cheaper and I felt more comfortable with a known supplier. However, I may have to wait 1-2 weeks. If it works for me, then a local supplier would be good, if you can tell me.
Hi Diane. It was by MZ. I planned to purchase it but now it’s suddenly unavailable.
I have just searched MZ in Amazon and google and can’t find it either. Remember to check out any company through TrustPilot before buying. Some products look as though they are UK but end up being sent from India and you will have a long wait. I hope you find one that you are happy with.
If looking for LOLA in USA, Nutricost & NOW both have capsules on Amazon. Life Extension has powder on Vitacost.
Court, can’t thank you enough for your reporting. I wouldn’t have known about liver involvement w/out Health Rising. I have advanced liver fibrosis, but enzyme tests are all goid. I was just diagnosed w/ excess ammonia and prescribed Lactulose syrup, which draws water into colon to help remove ammonia. Doctor said to note if it helps with energy & clear head—symptoms I always pinned in ME-CFS. Not getting my hopes too high, but it would truly be great if ridding ammonia made a significant difference. I already take ornithine in my Bullet Sleep supplement.
I manage physical activity well but notice that mental work really does me in. Eye exams are especially fatiguing with their extensive concentration (is #1 or #2, #2 or #3 better?) and I know to schedule nothing for a few days. Eager to hear more on Chris Armstrong’s work.
Thanks also, to commenters who mentioned “poisoned” feeling. Thought I was the only one & can’t explain it well enough to mention it to providers, but I associate it w/ low fluids & sometimes can actually feel it travelling down my arms.
Thanks and good luck:)
Is aspartate safe? Wouldn’t too much ammonia cause pH change in body?
Aspartate is increased in ALS patients and is an excitatory neurotransmitter… is it safe long term?
I am in US and just ordered this through my Amazon account.
BulkSuppements [I already use their d-ribose powder]
L-Ornithine L-Aspartate Powder – Supplement.
The Kilo pkg has over 300 servings for around $40. Various pkg sizes. I wanted to have plenty to share with friends.
I just checked out reviews on the source supplier BulkSupplements and found many documented negative recent reviews. I’m trying to find a good brand to try here in the US.
https://illuminatelabs.org/blogs/health/bulk-supplements-review
https://www.trustpilot.com/review/bulksupplements.com
Good luck all!
Be careful with supplements from Amazon as there’s a lot of counterfeit products often being found on there, as Amazon don’t analyse the products.
Also the brand NOW had discovered their own brand had been counterfeited and sold on Amazon, that didn’t even contain their claimed ingredients.
Amazon is the Wild West of supplements. You better to be buying from a company like iHerb who often check many of their products via an independent lab. It will state “iHerb tested” or “iHerb Brand’. Or at least you know the other brands that don’t say that a regulars to iHerb.
‘Fraudulent Products Sold on Amazon Impersonating Prominent Brands’ https://www.wholefoodsmagazine.com/articles/16086-fraudulent-products-sold-on-amazon-impersonating-prominent-brands
‘NOW’ got so upset with the fraud on Amazon that they started testing other random brands to show up Amazon fir the farce it is, and in doing so discovered the products under different brand names were worse than even they expected
‘NOW Testing of Bromelain Supplements Purchased on Amazon Reveals “Abysmal” Results | WholeFoods Magazine
https://www.wholefoodsmagazine.com/articles/16073-now-testing-of-bromelain-supplements-purchased-on-amazon-reveals-abysmal-results
Amazin reacts late in removing products too, but they admit counterfeit and impurity issues are happening
https://www.businessinsider.com/amazon-warns-customers-that-supplements-on-its-site-are-fake-2019-7
Hi myself I have a subscription to Consumer Labs that analyse and test many different brands. So after a while you get to see the brands that you trust. Which off the top of my head are…
Swanson,
NOW,
Dr’s Best,
Lake Avenue,
Thorne
Brenden, Thanks for the warning. I have heard this from many other sources and usually enver buy any vitamins or supplements on Amazon. I hope everyone at least listens to your advice because it truly is the wild west market. It’s my understanding that the two companies with the most sophisticated & safe production lines are THORNE and PURE ENCAPSULATIONS. The company i buy my V&S from is called FUll SCRIPT_ I have been advised by several functional medicine providers the Fullscript carries the safest and most effective V&S- they screen the companies they carry (and I think they only care about 10 brands.) ( I don’t work for any of these companies- just my opinion.)
I found LOLA on Amazon, $19.95 for 8.8 oz. I’m trying it and hopefully will see an effect when I take my dog for a ‘brisk” walk today! Happy 4th❗🎆
In the US you can find various powder / pill forms on Amazon – https://www.amazon.com/s?k=L-ornithine+L-aspartate&crid=D2GWCSIR5LUC&sprefix=l-ornithine+l-aspartate+%2Caps%2C285&ref=nb_sb_noss_2
Thanks Cort! This could make a difference for me! I’ll let you know.
Do we have any idea about dosing for this?
They didn’t provide any information on that. For me I would probably start out at a quarter of the recommended doses on the package and go up from there.
I Cort,
Interesting to know that I am not the only one very sensitive to everything and that usually recommended doses are always way too much for me…
Needing to start at low doses is very common – as are weird reactions to things! Daniel Clauw – FM researcher – thinks it has to do with a sensory system that has run amok.
Is there a difference between products which are labeled LOLA/bonded l-ornithine l-asparate and those just listing the substances? Are these chemically different? p.s.Thank you for all you do for us!
Be careful with supplements from Amazon as there’s a lot of counterfeit products often being found on there, as Amazon don’t analyse the products.
Also the brand NOW had discovered their own brand had been counterfeited and sold on Amazon, that didn’t even contain their claimed ingredients.
Amazon is the Wild West of supplements. You better to be buying from a company like iHerb who often check many of their products via an independent lab. It will state “iHerb tested” or “iHerb Brand’. Or at least you know the other brands that don’t say that a regulars to iHerb.
‘Fraudulent Products Sold on Amazon Impersonating Prominent Brands’ https://www.wholefoodsmagazine.com/articles/16086-fraudulent-products-sold-on-amazon-impersonating-prominent-brands
‘NOW’ got so upset with the fraud on Amazon that they started testing other random brands to show up Amazon fir the farce it is, and in doing so discovered the products under different brand names were worse than even they expected
‘NOW Testing of Bromelain Supplements Purchased on Amazon Reveals “Abysmal” Results | WholeFoods Magazine
https://www.wholefoodsmagazine.com/articles/16073-now-testing-of-bromelain-supplements-purchased-on-amazon-reveals-abysmal-results
Amazon reacts late in removing products too, but they admit counterfeit and impurity issues are happening
https://www.businessinsider.com/amazon-warns-customers-that-supplements-on-its-site-are-fake-2019-7
Hi myself I have a subscription to Consumer Labs that analyse and test many different brands. So after a while you get to see the brands that you trust. Which off the top of my head are…
Swanson,
NOW,
Dr’s Best,
Lake Avenue,
Thorne
I saw the study info released my OMF but have yet to drill down on the specific info. I’ve been found to have high serum ammonia levels in the past. As such, I am surmising this supplement may help me. What I’ve been interested to find is dosage protocol IF one were to try to replicate this? If anyone has this already I’d appreciate it. And if anyone knows of what brand is used in the study or any knowledge of reputable brands. This is such a specialized supplement. A quick search on Amazon has turned up some but no brands I’m familiar with.
I have spent ages hunting for LOLA here in the UK after reading the Open Medicine Foundation comment recently. I could only find hideously expensive capsules here (think over £50 for one bottle) and ended up buying bulk powders from USA. I felt it was worth a try. Fifteen years ago I got a huge boost in energy from phenylalanine (amino acid) but went badly downhill nearly 5 years ago after helping a neighbour. I am ready to try another amino acid. This has good explanations behind it. I think I will know if it’s helping by the time I finish the container (about 3 months).
Hi Diane
May I ask what brand and dosage of Lola you are using?
Very interesting area to follow up. May be worth a try for my partner.
Bulk supplements.com
I am unsure of dose. They speak of 3g daily. I think I will start with 1g and see how I go.
Thanks Diane.
Good luck with it I hope it helps
Hi Diane, what brand are you using please?
bulksupplements.com
Please let us know if it works for you.
Would love more information on their dosage and if it has any interactions with other medications. I sadly don’t have much brain power to read long articles, but this sounds interesting!
I’ve had my ammonia levels checked and they were fine. Is that relevant? Also, I’ve tried ornithine without any improvement. This is the first I’ve heard about L aspartate though.
Mine is arriving today. I got it from Amazon.ca.
I really appreciate you saying that that this is not a cure. Most of what I do as self treatment is aimed at reducing the symptoms so I can function more normally – which is happening. But I have no illusions that I have anything like a cure. It becomes very obvious when I over do activity.
BTW I can’t access my profile When I click the link it takes me a blog.
I’m confused where it says “Note that the authors do not suggest that L-alanine and L-ornithine supplementation will cure ME/CFS.” Isn’t the article talking about trying L-aspartate and L-ornithine? Or is there a suggestion that one should try taking L-alanine too?
Probably a typo! With all of these unknown names, I ordered the wrong supplement yesterday and only realised after opening the bottle today! It came up in an Amazon search for the right names. I saw a make I knew, two strange names beginning with l- and clicked on it. No. I now have a bottle of l-arginine and l-ornithine. (I am normally more savvy with my searches. It must have been a bad day because I also forgot to click on the free postage box, after being so careful to buy extra things to get my amount up to the free level.)
Hopefully there will be a follow-up study. I get so frustrated seeing all this great one-off research that doesn’t go anywhere. Right now it seems as though there is a different theory to what causes ME for every day of the month.
100% agree. Follow-through research on promising leads in ME/CFS has been really really poor.
Having said that, I am going to try this supplement rather than wait for further research trials. It’s worth a shot at least. It’s not too expensive and doesn’t have a concerning side effect profile. And at least there’s some scientific rationale behind it.
I am a little skeptical but who knows. My money is on these low amino acids being a by-product of the illness rather than a central driver. But I hope I am wrong, and they help.
My money is still on the brain as being the key to the illness. And that the complexity of the brain is why the illness has been so hard to ‘solve’. I have held this view since the Dubbo studies were done more than 15 years ago.
I did the same thing! Now I have a bottle coming with only one of the LOLA ingredients. I feel your frustration!
Oh no, Linda! Brain fog is real and expensive!
I wanted to emphasize that the authors are proposing that it may be able to ameliorate the core symptoms of ME/CFS – but they are not saying that it will cure ME/CFS.
Too many times I think we expect something to be a cure and then get disappointed when it’s not.
Yes but the study was about alanine & ornithine, yet you say they suggest LOLA which is aspartate & ornithine? Where has the alanine gone – should that be added to the LOLA? Its a bit confusing why aspartate is mentioned 🙂
Hey I’m confused about this too?
Even a 20-30% improvement would be good!
My doctor thinks it is worth trying these amino acids. It’s not prohibitively expensive and the amino acids seem to have a low side effect profile.
The paper does not postulate a causal mechanism for the findings. It would be interesting to think about what might be causing these low levels of amino acids. Inflammation? Immune system dysfunction? Or something else?
My money is still on the brain being the key factor in ME/CFS.
I bet inflammation
Cort, with the exception of LDN or Abilify. Is there any other supplements or off label drugs worth trying for CFS?
Thanks for writing this article C
The research results seem to be getting closer to solving the ME/CFS puzzle as they drill down to the various details. At the least, we seem to be getting closer to possible treatments that would actually improve functioning somewhat. I’d love to volunteer to take these supplements for an official study if it gets funded. I’m signed up in a general volunteer database (through OMF, I think), but there never seems to be any research programs close to me. I hope this possible treatment moves forward.
I agree. It’s encouraging that the LOLA idea resulted from a complex study of the molecular biology of ME/CFS! That’s pretty cool 🙂
From a quick scan, it seems that inflammation can reduce amino acids, yet amino acids can also reduce inflammation. So I guess supplementation is potentially ‘killing two birds with one stone’.
I was wondering how much to take. This study using it for hepatic encephalopathy used 18g per day in three divided doses, so I guess I’ll work my way up to that. I can’t find anything about side effects. Did you find any dosage information Cort? https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4131305/
No…except for the possible gut side effects LOLA seems pretty well tolerated. I think I would start at a quarter of the suggested dose on the container and work my way up from there.
In another part of the paper they talked about using 3g 3 times a day. That feels more reasonable to try
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4131305/
I would like some Mecfs practitioners to weigh in on what brand, dose we should be trying. I think L-ornithine L-aspartate might also be available in IV. I would love for example if Dr. Bonilla published (to his patients) newsletters with his opinion on some of these latest findings, how we might want to do any associated testing with our primary care doctors, and his recommendation for how to try these potential treatments. Perhaps Bateman Horne or INIM would help the community with useful information like that.
A treatment protocol would be nice, including how much to start with, how to increase, and when to decide it doesn’t work much for you.
Just saying ‘start low’ isn’t helpful enough, and talk of bloating and diarrhea as potential side effects sounds like a lot of the other things I’ve had to stop in the past.
I think I’ll wait to hear the results from people trying this – but an increase in energy can only help.
I always get the stupid side effects; makes me wary.
Hopefully they will follow up on this small study with something relatively larger. I’m sure once they have more information on how effective it is, they’ll then go ahead with recommending these supplements in general with dosage, side effects etc.
I’m sure once more people start using it they can give some pointers on where to find it & how much to use. I’ll be following also & waiting eagerly!
There seems to be some confusion regarding what is being suggested. James has noted this as well: “LOLA is the term used for the L-ornithine L-aspartate supplement. LOLA has been used to reduce ammonia levels in non-alcoholic fatty liver disease for decades. Note that the authors do not suggest that L-alanine and L-ornithine supplementation will cure ME/CFS. ” A clarification would be much appreciated. In one instance aspartate is reference, in another, l-alanine is referenced. l-ornithine is referenced in connection to both.
“
As I said above, I think this is a typo. Note that it occurs in the summary, not in the main body of the text. Note also that Cory had been talking about alanine just before that. Anyone who has done a lot of writing knows that sometimes you use the wrong word, one you have just used previously. If you are unsure still, click on the OMF link and see that they recommend LOLA.
Thank you. Yes. Those of us who do a lot of writing do understand that typos can happen. Its ok to double check for precisely this reason. And it is much appreciated how much time, work and grace Cort puts in to benefit so many of us.
Cort is definitely very much appreciated. Most of what I know about ME, I learnt from his blogs. (I just re-read my previous reply and see that my finger slipped to Cory instead of Cort. My fingers are too big for iPads!)
Our former neighbor’s wife died from liver failure two years ago, and suffered with hepatic encephalopathy beforehand. LOLA is used to treat HE from liver disease, as is Xifaxan, which she took. She was surprised to hear that I had taken Xifaxan in the past for SIBO that resulted in IBS, which many of us with ME/CFS/FMS suffer from as well. Makes me wonder if there’s a connection here.
I just made a similar comment! We have some remission stories by people in our ME/CFS community who takexifaxan longterm.
I personally had a temporary remission while on a 2-week course of it for SIBO/IMO. It was a glorious tease.
Did you see any improvements while on Xifaxian?
Oh, yes! When I took it over a decade ago, my SIBO was severe. I hadn’t gone gluten free and low FODMAP at that point, so Xifaxan really turned my gut health around. But now that I can control my gut dysbiosis with a better diet and probiotics like Saccharomyces boulardii, I don’t have SIBO issues that require Xifaxan. But it makes me wonder if taking it for ME/CFS would be of benefit? Just wish it was cheaper. Even with my drug plan, the co-pay is still several hundred dollars a month.
What is SIBO, please? ?
SIBO stands for Small Intestine Bacterial Overgrowth, which is a form of gut dysbiosis. It can be the result of IBS (Irritable Bowel Syndrome), which is common in fibromyalgia.
Thank you, Judi! I might look into this for myself.
JR…just to tell you a guy on youtube got rid of his SIBO by suplimenting with
Artichoke/ginger supliments.
I tried ginger/lemon in a food blender…my SIBO seems to be gone,also my irritable bowel syndrome has been coming and going and lots of noise going on all over my guts which seems to be waking up my movements.not sure how else to explain this.Definitely change for the better
Thanks for taking the time to tell me, and glad you found something that helps! Did you blend the lemon slices whole including peel? Cheers!
I’ve heard before that ginger is beneficial for digestion, and google says so too: https://www.hopkinsmedicine.org/health/wellness-and-prevention/ginger-benefits. I’ve sometimes nibbled on a bit of ginger when having trouble with digestion.
It is also a stimulant which some with ME/CFS might need to be mindful of.
2 slices of lemon ,peel ON
thumb sized pc of ginger peel OFF
blended then drained through filter
I’ll dig up the youtube video on artichoke/ginger and post below this.
I couldn’t find the supliments together.im not much of a searcher though
https://youtu.be/53f1gsRUxvY?si=bS5eaMJ1mHjho1Tb
SIBO cure
Thank you Buckey!
I just recently found a guy’s youtube video talking about how he took ginger and artichoke supplements for his SIBO. I didn’t have the artichoke but I started blending a thumb size ginger with 2 slices of lemon blended with one cup of water…strained and drank….my SIBO seems to have vanished after a couple weeks of this.i do it every morning
That’s interesting. The only thing I found that made a dent in my SIBO/IBS besides Xifaxan, is a gluten-free low FODMAP diet.
I’ve stayed GF for over a decade (tested positive for gluten antibodies), so that’s with me forever. But every time I’ve tried to put FODMAP foods back in my diet, here comes the SIBO/IBS. I can get away with eating them in very small quantities once in a while, but not every day. And that really sucks, because so many low carb healthy veggies and fruits are FODMAP. I’ve considered something like FODZYME digestive supplements, but they’re expensive.
Martin Pall’s perspective on the role of nitric oxide in CFS/ME pathology would seem to contradict these findings. For me, any problems I have with supplements, scrip meds, or activities can be traced back to a likely increase in NO. Both ornithine and aspartate are precursors for NO and can result in a NO increase. That’s part of the reason they’re popular with athletes. Any insights or comments about this appreciated!
I was also thinking about Martin Pall in this context. I think there may be a system of systems sort of paradigm that brings these seeming contradictions into the same picture. I am going to pull his book from my shelf and have a look and report back what I can figure out. But it may take me hours or even a day or so since I have been struggling lately. I hope to have my mind settled on this by the time my pkg arrives from Amazon tomorrow. I felt that intuitively this was going to be good for me to try but hope to work out the theory to explain why. And then track how it does for me. Will keep you posted. I hope. :-/
Hi Ruth Anne! I’d be really interested to hear how you get on and if you manage to figure the link sometime – thanks and take care
I just received my first bottle of LOLA today. Might wait for more comments on here in case anyone else has already tried this.
The good part is powder formulas allow for micro dosing which we tend to need!
Anyone else about to try it already tried this?
Xifaxan/Rifaximin is FDA approved for hepatic encephalopathy. It helps remove excess ammonia from the body!!!
It is also used for traveler’s diarrhea and SIBO/IMO.
We have some remission stories by people in our ME/CFS community who take it longterm. I personally had a temporary remission while on a 2-week course of it for SIBO/IMO. It was a glorious tease. This has me wondering…
Hi Rachel,
Are those 2 supplements you’ve mentioned here the same/similar to the supplements mentioned in this article? As I would consider trying them to improve symptoms but I’m in the UK and I think a few people are having problems sourcing them.
TIA 😊
Donna
I dropped the hint about LOLA in my comments on Cort’s last blog, and Cort I am very glad you picked up the ball and made comments so quickly!
I have already placed an order for LOLA (powder) with BulkSupplements.com. I have ordered from them in the past and so know they are a reputable U.S. company–and not so expensive.The site is mostly for body builders so be prepared to be bombarded with muscle ads! 😉
I have suspected that amino acids might be helpful in ME/CFS and so already have been taking Liposomal+ Multi Amino powder (Centurion/Codeage). It has been helping but not earth shattering results. I suspect that by adding these compliments I can help my stamina bit by bit. I have tried Oxaloacetate in the past but it was just too expensive for the below modest improvement (sometimes hard to tell–which is common with many of the things I try).
One thing I notice with some of these studies is the eye popping doses that are sometimes used. Not sure what to dose with this one, but will research more and titrate up. Always good to try things for at least several months before the reject or accept pile. I’ll report back with the results sometime in the future.
I’ve had very good results with proteolytic enzymes and scar tissue (old acne scars), so I am ever encouraged.
I did try Efthymios liver supplement a while ago but dropped it because I didn’t notice much. Anyway, it mostly applies to him, not me. So everyone, best of luck with this one!
Thanks for cluing me into it Nancy :). There are several things I liked about the study. I always love to see ME/CFS org funded studies, then there was the multifactorial nature of it – combining different data sets together which I think is crucial – and finally a treatment recommendation that is readily available 🙂
Trust pilot reviews are bad for Bulk Supplements claiming there are undesirable fillers. I’m going to try Allergy Research Group’s product.
Re Bulk Supplements brand, see also analysis of lab test results … not so good! See the comments after the lab results. Seems they are trying to improve.
https://illuminatelabs.org/blogs/health/bulk-supplements-review
I found a small company that seems to strive for quality. I will follow up after I try their product.
Good luck to us all!
LL-aspartate has a neurotransmitter function.
It affects the neuronal functions of the brain, memory, cerebral activity and spatial orientation.
L-aspartate could also play a role in testosterone synthesis and could be of interest in men suffering from low testosterone levels.
(Besides, in fitness, certain ‘testosterone booster’ food supplements contain aspartate).
Cort talked about testosterone in an article a while ago, and that perhaps it was low in men and women in ME/CFS and could play a role?
Probably. My free testosterone was low and SHBG was sky high which usually indicates problems in liver detoxification
Interesting. I am female with low testosterone, had thought about trying boron for it (if I remember correctly).
Cort, Thank you for mentioning my story and the association of liver function and LOLA.I believe that the work from Wenzhong Xiao and his team may have also identified other potentially important associations with findings from the AI system I have been using which -back then- appeared to be irrelevant. Thanks to Wenzhong’s work and his team more pieces of the puzzle may now be coming into place.
I winder how many people with CFS have had abnormal liver function? I had slightly poor liver function for the first three years of the illness, when my health was at its worst
I
I was diagnosed with non-alcoholic fatty liver disease.
Thanks for the fantastic reporting as usual. I have noted a strong ammonia smell in my urine during PEM for years and this probably explains why. I’m one of those people who has benefited tremendously from oxaloacetate, (I have recovered about 50% of function) I’m looking forward to trying this as well.
Good to hear that oxalo is helping! I came across a Facebook post where both a woman and her daughter benefited tremendously. As I remember one recovered.
Do you know if you have high protien from a urine test….I do…which I’ve read indicates infection
Hello Brian, came back to check on Lola experiences and saw your post. The oxaloacetate, how does one procure it? Would you have a few brand names? I don’t think it exists as a supplement in Germany, so I might try and import it. Cheers and best wishes for further recovery!
There is only company that makes it, should be easy to find online. The sell a low dose but that is the wrong one. You want to buy their high dose medical supplement. Send me an email and I will send you a link. I’m easy to find, just look up Cape Falcon Kayak online
Hi JR,
I am in Germany and ordered the L- ornithin L-aspartat as capsules from
http://www.wellnest-shop.com
They are pricey,but don’t have bad fillers etc.
Thank you, thank you, thank you to Geoff for the narrations.
Isn’t aspartate neurotoxic? Doesn’t it increase glutamate? Why would we risk taking it in a supplement?
For all the reasons the blog states and because many people benefit from using it as a supplement.
Actually a lot of people have major regrets after taking this as a supplement for significant periods of time. But you obviously didn’t know that.
No I hadn’t heard that.
Jason, …Do you have written sources of info on this and/or its cosiquences
I just ordered some
Thanks for completing the picture, could you please write more about this?
Hi Jason, I have tried so many things that made me worse. But, I am always hopeful for the next possibility. And some things have worked so they stay in my arsenal of treatments: Immunopro; bulouke; COQ10; B12; Quercetin; Nexavir; Cell Signaling Factors; clonazepam and natural progesterone. Nexavir is an antiviral in cream form and I tolerate it much better than Valtrex.
Can you give some references for people being made worse by treatment with these amino acids?
Hey Jason, I am not sure LOLA agreed with me and would like to compare with other people’s experiences. D’you remember where you heard or read about these experiences?
Though I can’t tell for sure due to mixing with other stuff, I suspect Lola gave me increased brain fog and sedation. It might fit with your other comment that it increases glutamate.
Thanks so much! Kind regards
Hi Cort, I looked a bit into the things Jason mentioned, see my new post below.
Wouldn’t fibromyalgia muscle weakness and exercise intolerance be related with these other conditions?
I would not be surprised…
Im confused here if there is a suggestion to try L aspartate, L ornithine only, or also to add alanine?
Firstly, from little New Zealand, I want to say a deep ‘thank you’, Cort for all the work you have done and continue to do. These little gems of research offer rays of light and hope.
I have a few questions:
– You refer to a previous study that looked at amino acids. I feel that there might have been a few studies in the late 90s/early 00’s. I recall, amongst others, Japanese studies
– I do wonder if we face the same issue here; that low levels of certain amino acids may be a by-product of the disease, not a key driver. Of course I hope this latest finding has clinical relevance. If many could take these amino acids and get at least some improvement, that would be great
– if the findings are not of clinical benefit, then I am sure they will be of benefit in studying the illness/s and hopefully lead us more quickly to causative mechanisms
– re: Chris Armstrong’s work : it is very interesting, are you aware of progress on the studies?
Thanks!
Also re: Chris Armstrong – I see his 2015 study found low levels of ornithine. So there really could be something in this
Right – they don’t attempt to explain why this might be I don’t think. There is the preference for amino acid utilization but why that’s happening is unclear as well as why these particular pathways might be disturbed.
I hope to interview Chris in our “What’s Up Doc?” series. He’s got a bunch of really interesting studies going.
I have started 500 mg l ornithine l aspartate twice daily. So 1 gram daily.
Will keep you updated.
I am severe 100% bedboumd like whitney.
Hi Anish, would love to hear how you get on sometime if you’re able? I’m severe and practically bedbound too – thanks!
Firstly, I am so grateful for this healthrising team and community.
Question: I find it hard to keep track of the different things I can try to improve my ME, is there a good resource / spreadsheet or something that others use? I want to be systematic but am not a highly organized person.
Thanks
Sherri Rogers(spelling?)m.d. in New York state wrote about pathways
Being blocked in her book(s)….that was 35 yrs. Ago!!!
She has books she has written about this illness, her being a sufferer….again, 35 or so years ago.
I wonder if she is aware all this science is being relived.
i have one of her books titled “environmental illness” another one of her books is titled ” detox or die”
The medical “community”
Didn’t care for what she was writing books and treating her patients
Another early crusader was Majid Ali…again (spelling?)
https://www.environmentalhealth.ca/w9394sherry.html
☝️this is what Sherry
Rogers m.d. war writing about way back in 1993….the year all hell broke loose with my immune system.i had no idea a person could become this sick
Gong-Hua Li’s preprint states that “the most significant metabolic change in ME/CFS and Long COVID patients was the downregulation of alanine and aspartate metabolism.”
But Fredrik Hoel and Øystein Fluge [he’s the brilliant ME/CFS metabolic researcher] study found aspartate levels and glutamate levels were significantly *higher* in patients with long COVID and ME/CFS than in healthy controls. [link below]
So with this contradiction in measurements and conclusions, there’s not clarity on if there’s an excess or a deficit of aspartate or glutamate in pwME.
Since aspartic acid/aspartate is in every cell in the body and important for energy creation —
it’s the precursor to oxaloacetate, which is low in in pwME — and to methionine (metabolism), threonine (CNS), isoleucine (energy) and lysine (energy from fatty acids),
could aspartic acid supplementation help? Or make things worse?
At what point do high levels of aspartate and glutamate become neurotoxic?
Are high levels of these in PWME far away from that neurotoxic threshold?
Oxaloacetate deserves a larger trial — at higher doses for a longer time — to determine if the benefits physicians are seeing clinically at higher dosages can be quantified. Perhaps trial oxaloacetate against dichloroacetate and a control since the dichloroacetate is much less expensive.
The Holy Grail, of course, would be to go upstream to where and how the citric acid cycle gets broken in the first place, from virus damage to the mitochondria and the shutdown of the mitochondria entirely in infected cells by the innate immune system.
I’d like to see far more research on measuring viral replication of ME/CFS viruses in the brain (like the research that found actively replicating SARS2 virus in the brain of Long-COVID patients), and clinical trials of the newer antivirals and monoclonals to kill those viruses
and stop all the downstream disorders that result from the brain’s viral infection.
Aspartate levels and glutamate levels higher — not lower — in patients:
“A map of metabolic phenotypes in patients with myalgic encephalomyelitis/chronic fatigue syndrome,”
Fredrik Hoel and Øystein Fluge [whose ME/CFS metabolic research I admire very much]
from Haukeland University Hospital, Bergen, Norway.
https://insight.jci.org/articles/view/149217
Gosh that is complex!
Where does it show aspartate levels are higher? Isn’t the study showing that levels of various things vary depending on subtype?
But it’s all beyond me!
And here’s a study published April 2024 finding higher levels of glutamate and n-acetyl-aspartate in the brains of ME/CFS and long covid!
https://www.amjmed.com/article/S0002-9343(24)00216-X/fulltext
Is the n-acetyl-aspartate in this study a different metabolite to the aspartate measure in the study featured in Cort’s article? Could levels be high in the brain, and lower elsewhere?
Again, confusing and way beyond my knowledge!
The higher brain glutamate levels is interesting, and makes a lot of sense.
I am looking forward to seeing more brain research come through. Maybe a Younger paper in 2024?
I wonder what kind of ammonia are we speaking of.
There lots of anhydrous
Ammonia being sprayed on crops
Or is it ammonia being produced by our own bodies?
If, as indicated in the comments, there are conflicting findings in studies as to whether l-aspartate is lowered or elevated in me/cfs, perhaps it’s worth trying just supplementing with l-alanine and l-ornithine?
Yes. The conflicting findings regarding l-aspartate are troubling. In addition, Gong-Hua Li’s research references those with Long Covid while that of Fredrik Hoel and Øystein Fluge does not. For those who have Long Covid, this is of particular significance and point to the question of whether and how those conflicting findings are relevant to the recommendations made. I’m wondering if anyone knows of any professionals who could provide insight and guidance regarding this.
I’m surprised neither of the teams has commented on the conflicting findings – I understand that both groups are associated with the Open Medicine Foundation so I would have thought there’d be some contact between them. I think the relevant sentence in the Fluge paper is “Affected amino acid metabolites linked to pathways of alanine/aspartate/asparagine, methionine/cysteine/taurine, lysine, glutamate/glutamine, histidine, and phenylalanine, generally expressed higher levels than in the HC group”, so they are not saying directly that these pathways have higher levels, but only that ‘affected amino acids linked to pathways…’ – perhaps that’s significant??
Anyway I’m going to try taking ornithine (there’s research that shows ornithine alone helps fatigue in healthy people… https://www.sciencedirect.com/science/article/abs/pii/S0271531708001929?via%3Dihub ), and perhaps also take l-alanine (or beta alanine??), even though that’s one of the pathways that’s mentioned in the Fluge paper – but at least l-alanine isn’t an NMDA agonist (unlike l-aspartate), so it won’t cause any problems there.
Geez, nothing’s simple with this disease! It’s so difficult trying to work out what’s going on and what to do!
I notice that Mara below is trialling LOLA – if she and others have a lot of positive feedback I will give it a go.
Thank you for this, George. A very thoughtful and compassionate response. I’ve not noted any reference to the question of long-term effects of LOLA nor how long one could safely take it. So that is a bit of a concern. I did notice a study using LOLA for four months on patients with cirrhosis of the liver. But that is not what is being addressed here. Would love to hear more of your use and dosage of l-ornithine as you trial that.
Ok, Lise, I will let you know how I get on with the l-ornithine and dosage etc. 🙂
sorry in line 11, I should have written ‘affected amino acid metabolites linked to pathways…’
Thanks for writing this in depth article about the study! I went ahead and bought some LOLA right after this study was released. Judging on comments here, lots of other patients are doing the same. In case anyone is interested: I started out with 3grams and quickly found out that was too much to start with! It upset my stomach (some diarrhea) so I tried about 1-1.5 grams in the morning. I noticed some queasiness but not much other side effects. After about 4 days I had a noticeable increase in energy. It’s been a week now and I’m feeling about 20% increase in energy and much clearer mind (no brain fog). Now whether this will keep up and if I can increase the dosage, time will tell. But promising so far! I am being super cautious to not push myself too much too soon and cause a big crash. I wish everyone luck and hope this can help! (Fwiw I took a chance and bought the cheaper brand of Bulk Supplements. Maybe next time I’ll try a more expensive brand to compare).
Many thanks for the feedback! I’m thinking of trialling LOLA myself, so I am following this with interest.
Just a note about LOLA from Bulk Supplements: I’ve found numerous warnings about this company and the lack of quality and oversight and misrepresentation of ingredients. Concerning. Given how very expensive this is when sold by Allergy Research, I’m wondering whether there is a way to purchase l-ornithine and l-aspartate as individual supplements in dosages that would be equivalent to what would be found when combined.
Oh dear – I ordered some supplements from Bulk Supplements, as they seemed very reasonably priced.
I accidentally clicked on the link that said “click here to stop receiving these messages”. Do you or anyone know how to undo that?
Regarding Bulk Supplements: Wishing you a good experience with that. I am leery of companies that have such a extended set of warnings from a range of sources. That said, I hope all is good moving forward.
Hi George! I meant to reply to your comment but I think I replied in the wrong place? so much for my improvements in brain fog! Lol You should be able to see my response on here somewhere xD
I hope you’ve had a chance to try it out! So it’s been about 5 weeks now of taking LOLA and I’m definitely having good results. I’ve slowly increased up to 1.25 teaspoons (aprox 2.5 grams) without any negative side effects. I would say I have aprox 20% increase still in energy overall but biggest change I’ve noticed is in my mental state. Less brain fog and generally feeling more positive. For me that is a major improvement! It’s definitely not a cure so I would encourage anyone to exercise caution and not push too hard even if feeling a lot better. I’ll keep slowly increasing to see what happens. Hopefully this will continue to work but I’m always cautious.
I thought it worth mentioning that L-Arginine (with Vitamin C) has been trialed quite successfully for Long COVID. Arginine is a precursor to Ornithine, apparently.
See the 2022 paper by Izzo et al:
https://www.sciencedirect.com/science/article/pii/S104366182200305X
I am trialing with 2 grams(500 mg tabs X 4 times a day) from 7 days. No changes yet but I think I have less pain and distorted sleep.
Energy you can say 1% IMPROVED. but it’s early and hard to tell. I will keep updated everyone keep checking.
Many thanks for the feedback 🙂
I found a nice liquid version of LOLA, just $10 for a 30 day supply. I started at 1 gram and am up to 1.5. Two weeks now and I am experiencing improved symptoms of fatigue and brain fog. I tried oxalacetate earlier this year and had NO change in symptoms, and it was hella expensive.
Big thumbs up on this! I know so many of my pathways are broken, so it’s hard to know where to start sometimes. Many of you have so much knowledge, I’m glad I’m surrounded by such a smart group.
Sidenote-:I also have non alcoholic fatty liver and sometimes have a faint ammonia smell in my sweat. This smell is now gone, and it’s been over 90 this week in the Midwest.
Can you share the brand or source of the liquid LOLA? I tried looking for that online but couldn’t seem to find it. I’ve been using the Bulk Supplements brand in powder.
Wave Drops Liver Support Liquid… https://www.amazon.com/dp/B0CJVNSB9B?ref=ppx_pop_mob_ap_share
Thanks for the link! If I’m calculating right though the dosage listed for the product is 250mg of LOLA per serving (125mg each per 2ml)? That is 1/10 of what I’m taking now (2.5 grams or 2500mg). Are you taking 5x the 2ml dose a day? Or am I missing something?
Oh gosh no. Directions on the bottle say 2 ml daily. I started at 1 ml and have worked up to 2 ml.
I accidentally clicked on the link that said “click here to stop receiving these messages”. Does anyone know how to undo that?
I tried LOLA at 2g twice a day. A serving is 3g, so 1 1/3 servings per day. I have these weird once a month PEM relapses that don’t seem related to too much activity. LOLA didn’t stop the relapse this month, but did eliminate my muscle pain during the relapse. I still had my fatigue and neurological symptoms and POTS increase. But I’m wondering if LOLA will protect my muscles from PEM damage. I don’t know what causes my relapses, every 25 days when I do the same activity those days (~750 steps). I’m post-menopausal, and tried hormones which didn’t help. I’ll keep taking LOLA. Maybe some healing will take place that will help my other symptoms.
I would rule out 2 illnesses that are linked to liver disease & a lot have both conditions combined.
Alpha 1 Antitrypsin Deficiency & Iron Overload hemochromatosis, some are only genetic carriers these are ones who likely have anemia or pernicious anemia.
It’s better to be safe than sorry. Genetic COPD goes with Alpha 1.
Shortness of breath/chronic fatigue are some of the symptoms xx
Anyone who has been experimenting w/ these supps want to report their results? @Cort — have you heard anything from ppl who are trying them?
I have been using it about 5 weeks and having some good results. You should be able to see my reply earlier on a previous comment with more details. Hope this helps!
I have been on LOLA since this story broke and nothing improved as usual!
I tried lola and had an amazing reaction for two days felt normal almost….but did way too much and crashed for a week or more. Trying again but pacing regardless this time. I think it is giving my stomach issues but worth it if improvemeht in energy. Anyway hoping it will help.
Has anyone found L-ornithine L-aspartate combination or L-aspartate alone in a capsule form, not a powder? One available to buy from U.S. Please post a link if you have. Thanks!
I just received my Ornithine 500 mg and Aspartic Acid 500 mg from a compounding pharmacy. My CFS Dr in NYC prescribed this for me as part of this study. I started taking the supplements 3 days ago. I live in NJ and the compounding Pharmacy is located in MA.
Oh that’s great! If you don’t mind sharing, which pharmacy and what is the cost? Wondering if it is comparable to buying both supplements separately or cheaper. Thank you!
Erika, the pharmacy is called Johnson Compounding & Wellness. They’re located in Waltham MA. The cost for the 2 (30 day supply) was pricy. Over $100 but I felt more confident with a pharmacy rather than Amazon. And my Dr. uses them.
I tried LOLA only briefly (1g once, 2 g once). Though I can’t be sure if the effects relate to LOLA only since there was other stuff in the mix (might have been drug interactions), I suspect that LOLA gave me increased brain fog (mushy brain) and had sedative effects.
Though the medical leaflets for the registered LOLA medications for hepatic disease mention very few side effects, I am not convinced that LOLA may be safe for everyone, due to the following reasons:
1) I looked up and (without going deeper into those studies) could confirm there is literature on what Jason mentioned above, i.e. aspartate neurotoxicity https://pubmed.ncbi.nlm.nih.gov/2746696/, aspartate influencing glutamate synthesis https://pubmed.ncbi.nlm.nih.gov/2746696/, and in particular I also found this “Glutamate becomes neurotoxic via the N-methyl-d-aspartate receptor when intracellular energy levels are reduced https://www.sciencedirect.com/science/article/abs/pii/0006899388907652 .
2) This https://pubmed.ncbi.nlm.nih.gov/32424601/ also mentions that when applied directly to incubated brain slices from guinea pigs, L-Ornithine produced significant “sedative” effects on brain slice metabolism, most likely via conversion of ornithine to GABA”. – However, when injecting guinea pigs with l-ornithine, l-aspartate and LOLA respectively, the same study found no brain effects from either of these substances beside a DEcrease of brain cortex glutamate from -ornithine; “most likely due to rapid metabolism of aspartate before reaching the target tissue”. It concludes that in guinea pigs, “LOLA has minimal impact on healthy brain energy metabolism due to systemic clearance and the blood – brain barrier.”
The point I am making is I’m not sure the blood brain barrier is healthy in ME/CFS (?) and there are definitely cellular energy problems present in ME/CFS. Also, without testing individually, we can’t know if every ME/CFS patient actually has enhanced lactate and ammonia levels (I can’t remember if they were any different in the brain and blood though), and even if they do, might ammonia levels be more variable in ME/CFS dependent on varying levels of exertion?
So the point I’m making is, if we would take this on a day with comparatively low ammonia, plus with a potentially leaky blood-brain barrier and in the presence of intracellular energy problems, might we be accidentally overdosing LOLA or possibly experiencing neurotoxicity via aspartate-related glutamate increase in the brain? (though I wonder if the glutamate decreasing effect of ornithine mentioned above could counterbalance).
The official leaflet of a German registered LOLA medication (HEPA-Merz) also mentions not to take LOLA in the presence of a more pronounced renal insufficiency, with serum creatine > 3 mg/100 ml as a recommended guideline. (“HEPA-Merz darf nicht eingenommen werden…wenn Sie unter stärkerer Nierenfunktionsstörung (Niereninsuffizienz) leiden. Als Richtwert kann ein
Serumkreatininwert über 3 mg / 100 ml gelten.”)
Maybe it’s a possibility to test for one’s blood ammonia and lactate?
i’m not sure LOLA its OK for me. Same, Increased brain fog and sedative state
I think I might misremembered the amounts held by the dosage spoon – dosage was probably rather 500mg and 1000 mg respectively (not 1 and 2 grams),